The choice of the points of melting ice and boiling water as the main points of the temperature scale is completely arbitrary. The temperature scale obtained in this way turned out to be inconvenient for theoretical studies.
Based on the laws of thermodynamics, Kelvin managed to construct the so-called absolute temperature scale (it is currently called the thermodynamic temperature scale or Kelvin scale), completely independent of either the nature of the thermometric body or the selected thermometric parameter. However, the principle of constructing such a scale goes beyond school curriculum. We will look at this issue using other considerations.
Formula (2) implies two possible ways to establish a temperature scale: using a change in pressure of a certain amount of gas at a constant volume or a change in volume at a constant pressure. This scale is called ideal gas temperature scale.
The temperature determined by equality (2) is called absolute temperature. Absolute temperature? cannot be negative, since there are obviously positive quantities on the left side of equality (2) (more precisely, it cannot have different signs; it can be either positive or negative. This depends on the choice of the sign of the constant k. Since the temperature of the triple point has been agreed upon considered positive, then the absolute temperature can only be positive). Therefore, the lowest possible temperature value T = 0 is the temperature when the pressure or volume is zero.
The limiting temperature at which the pressure of an ideal gas vanishes at a fixed volume or the volume of an ideal gas tends to zero (i.e., the gas should be compressed into a “point”) at a constant pressure is called absolute zero. This is the lowest temperature in nature.
From equality (3), taking into account that
![]()
flows out physical meaning absolute zero: absolute zero is the temperature at which the thermal translational motion of molecules should cease. Absolute zero unattainable.
IN International system units (SI) use the absolute thermodynamic temperature scale. Absolute zero is taken as zero temperature on this scale. The temperature at which water, ice and water are in dynamic equilibrium is taken as the second reference point. saturated steam, the so-called triple point (on the Celsius scale, the temperature of the triple point is 0.01 ° C). Each unit of absolute temperature, called Kelvin (symbolized by 1 K), is equal to a degree Celsius.
= .Any measurement requires the presence of a reference point. Temperature is no exception. For the Fahrenheit scale, this zero mark is the temperature of snow mixed with table salt; for the Celsius scale, it is the freezing temperature of water. But there is a special temperature reference point - absolute zero.
Absolute temperature zero corresponds to 273.15 degrees Celsius below zero, 459.67 below zero Fahrenheit. For the Kelvin temperature scale, this temperature itself is the zero mark.
The essence of absolute zero temperature
The concept of absolute zero comes from the very essence of temperature. Any body has energy, which it releases to the external environment during heat transfer. At the same time, body temperature decreases, i.e. less energy remains. Theoretically, this process can continue until the amount of energy reaches such a minimum that the body can no longer give it away.
A distant harbinger of such an idea can already be found in M.V. Lomonosov. The great Russian scientist explained heat by “rotary” movement. Consequently, the maximum degree of cooling is a complete stop of such movement.
According to modern concepts, absolute zero temperature is a state of matter in which molecules have the lowest possible energy level. With less energy, i.e. at a lower temperature, no physical body can exist.
Theory and practice
Absolute zero temperature is a theoretical concept; it is impossible to achieve it in practice, in principle, even in scientific laboratories with the most sophisticated equipment. But scientists manage to cool the substance to very low temperatures, which are close to absolute zero.
At such temperatures, substances acquire amazing properties that they cannot have under ordinary circumstances. Mercury, which is called "living silver" because it is in a state close to liquid, becomes solid at this temperature - to the point that it can be used to drive nails. Some metals become brittle, like glass. Rubber becomes just as hard and brittle. If you hit a rubber object with a hammer at a temperature close to absolute zero, it will break like glass.
This change in properties is also associated with the nature of heat. The higher the temperature of the physical body, the more intense and chaotic the molecules move. As the temperature decreases, the movement becomes less intense and the structure becomes more orderly. So a gas becomes a liquid, and a liquid becomes a solid. The ultimate level of order is the crystal structure. At ultra-low temperatures, even substances that normally remain amorphous, such as rubber, acquire it.
Interesting phenomena also occur with metals. The atoms of the crystal lattice vibrate with less amplitude, electron scattering decreases, and therefore electrical resistance decreases. The metal acquires superconductivity, practical use which seems very tempting, although difficult to achieve.
Temperature is a macroscopic parameter characterizing the state of thermal equilibrium of a system of bodies: all bodies of the system that are in thermal equilibrium with each other have the same temperature.
If the temperatures of the bodies are different, then when they come into contact, an exchange of energy will occur. A body with a higher temperature will give energy to a body with a lower temperature. The temperature difference between bodies indicates the direction of heat exchange between them.
To measure temperature use thermometers. Thermometers use the dependence of the volume of liquid (mercury or alcohol) on temperature.
When calibrating a thermometer, the temperature of melting ice is usually taken as the reference point (0); the second constant point (100) is considered the boiling point of water at normal atmospheric pressure. The segment between 0 and 100 is divided by 100 equal parts, called degrees. Based on this Celsius.
Temperature measured in 0С, denoted by the letter t.
There is also another scale - the Kelvin scale (absolute temperature scale).
Zero temperature on this scale corresponds to absolute zero, and each unit of temperature is equal to a degree on the Celsius scale.
Absolute zero- this is the limiting temperature at which the pressure of an ideal gas goes to zero at a fixed volume or the volume of an ideal gas tends to zero at a constant pressure.
Absolute zero corresponds to temperature t=- 2730C.
Temperature , measured in Kelvin (K), is designated by the letter T.
We have the largest information database in RuNet, so you can always find similar queries
This topic belongs to the section:
Physics. Answers to the exam
Physics answers. Mechanical movement. Evaporation of liquids. Special theory of relativity. Radiotelephone communication. Laws of dynamics. Electricity. Law universal gravity. Body impulse. Kinetic and potential energy. Oscillatory movement. Molecular kinetic theory. Temperature. Nucleus of an atom.
This material includes sections:
Mechanical motion and its relativity. Frames of reference. Speed and displacement during linear uniform motion
Evaporation of liquids. Saturated and unsaturated pairs. Air humidity and its measurement
Basics of service station. Inertial reference systems. The principle of relativity. Postulates of the special theory of relativity
Principles of radiotelephone communication. Amplitude modulation and detection. The simplest radio receiver
Force. Addition of forces. Newton's laws of dynamics
Electric current in solutions and melts of electrolytes. Law of electrolysis. Application of electrolysis in technology
The law of universal gravitation. Gravity. Body weight. Weightlessness
Electrical capacity. Capacitor and its structure. Energy of a charged capacitor. Application of capacitors in technology
Body impulse. Law of conservation of momentum. Jet propulsion
Rutherford's experiments on α-particle scattering. Nuclear model of the atom. Bohr's quantum postulates
Kinetic and potential energy. Law of conservation of energy of mechanical processes
Electric current in metals. Resistance of a metal conductor. Resistivity
Wave properties of light
Forced vibrations. Resonance. Dependence of the amplitude of oscillations on the frequency of the driving force
The astronomical yearbook gives the coordinates of the Sun, Moon, major planets solar system and stars, as well as other ephemeris quantities for specific points in time selected.
Sociology. Sociological thought in Russia
Sociology is the science of general patterns formation, functioning and development of society as a whole, as well as social communities and social relations. Sociological thought in Russia is developing as part of global sociological science.
Procedure for uncoupling a locomotive from a train
The procedure for uncoupling operating locomotives in trains of increased weight and length, as well as uncoupling from trains of operating locomotives that are traveling to part of the section, and the conditions for their circulation, ensuring traffic safety, are established by the head railway. Responsibilities of an assistant driver when attaching to a train
Obstetrics
Tasks of a nurse. Observation and assistance during childbirth. Reception and sanitization women in labor Birth injuries. Postpartum period. Nephropathy of pregnancy. Ectopic pregnancy. Premature detachment. Childbirth. Features of childbirth. Clinical picture. Etiology. Treatment. Obstetric operations. Diseases of the uterus.
Usna folk creativity, illumination and writing
Galicia-Volinsky chronicle. Architecture and location.. Painting, artistic crafts. Visnovki. Western Ukrainian lands, Galicia and Wolin
Absolute temperature zero corresponds to 273.15 degrees Celsius below zero, 459.67 below zero Fahrenheit. For the Kelvin temperature scale, this temperature itself is the zero mark.
The essence of absolute zero temperature
The concept of absolute zero comes from the very essence of temperature. Any body has energy, which it releases to the external environment during heat transfer. At the same time, body temperature decreases, i.e. less energy remains. Theoretically, this process can continue until the amount of energy reaches such a minimum that the body can no longer give it away.
A distant harbinger of such an idea can already be found in M.V. Lomonosov. The great Russian scientist explained heat by “rotary” movement. Consequently, the maximum degree of cooling is a complete stop of such movement.
According to modern concepts, absolute zero temperature is a state of matter in which molecules have the lowest possible energy level. With less energy, i.e. at a lower temperature, no physical body can exist.
Theory and practice
Absolute zero temperature is a theoretical concept; it is impossible to achieve it in practice, in principle, even in scientific laboratories with the most sophisticated equipment. But scientists manage to cool the substance to very low temperatures, which are close to absolute zero.
At such temperatures, substances acquire amazing properties that they cannot have under ordinary circumstances. Mercury, which is called "living silver" because it is in a state close to liquid, becomes solid at this temperature - to the point that it can be used to drive nails. Some metals become brittle, like glass. Rubber becomes just as hard and brittle. If you hit a rubber object with a hammer at a temperature close to absolute zero, it will break like glass.
This change in properties is also associated with the nature of heat. The higher the temperature of the physical body, the more intense and chaotic the molecules move. As the temperature decreases, the movement becomes less intense and the structure becomes more orderly. So a gas becomes a liquid, and a liquid becomes a solid. The ultimate level of order is the crystal structure. At ultra-low temperatures, even substances that normally remain amorphous, such as rubber, acquire it.
Interesting phenomena also occur with metals. The atoms of the crystal lattice vibrate with less amplitude, electron scattering decreases, and therefore electrical resistance decreases. The metal acquires superconductivity, the practical application of which seems very tempting, although difficult to achieve.
Body– this is one of the basic concepts in physics, which means the form of existence of matter or substance. This is a material object that is characterized by volume and mass, sometimes also by other parameters. The physical body is clearly separated from other bodies by a boundary. There are several special types of physical bodies; their listing should not be understood as a classification.
In mechanics, a physical body is most often understood as a material point. This is a kind of abstraction, the main property of which is the fact that the real dimensions of the body can be neglected for solving a specific problem. In other words, a material point is a very specific physical body that has dimensions, shape and other similar characteristics, but they are completely unimportant in order to solve the problem at hand. For example, if you need to calculate the average speed of an object on a certain section of the path, you can completely ignore its length when solving the problem. Another type of physical body considered by mechanics is an absolutely rigid body. The mechanics of such a body are exactly the same as the mechanics of a material point, but additionally it has other properties. An absolutely rigid body consists of material points, but neither the distance between them nor the distribution of mass changes under the loads to which the body is subjected. This means that it cannot be deformed. To determine the position of an absolutely rigid body, it is enough to specify a coordinate system attached to it, usually Cartesian. In most cases, the center of mass is also the center of the coordinate system. In nature, an absolutely rigid body does not exist, but for solving many problems such an abstraction is very convenient, although it is not considered in relativistic mechanics, since with movements whose speed is comparable to the speed of light, this model demonstrates internal contradictions. Absolutely the opposite solid body is a deformable body whose particles can move relative to each other. There are special types of physical bodies in other branches of physics. For example, in thermodynamics the concept of an absolutely black body was introduced. This is an ideal model, a physical body that absorbs absolutely all electromagnetic radiation that hits it. At the same time, it itself may well produce electromagnetic radiation and have any color. An example of an object that is closest in properties to an absolutely black body is the Sun. If we take substances that are common beyond the Earth, we can recall soot, which absorbs 99% of the radiation that hits it, except for infrared, which this substance copes with absorption much worse.
Video on the topic
Sources:
- Livanova A. Low temperatures, absolute zero and quantum mechanics
Temperature is a quantitative measure of the “warmth” of a body. The concept of temperature occupies a special place among the physical quantities that determine the state of the system. Temperature not only characterizes the state of thermal equilibrium of a given body. It is also the parameter that takes the same value for any two or more bodies that are in thermal equilibrium with each other, i.e. characterizes the thermal equilibrium of a system of bodies. This means that if two or more bodies having different temperatures, bring into contact, then as a result of the interaction between molecules these bodies will take on the same temperature value.
The molecular kinetic theory makes it possible to clarify the physical meaning of temperature. Comparing expressions (2.4) and (2.7), we see that they coincide if we put
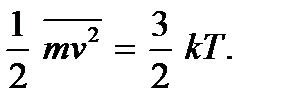 (2.9)
(2.9)
These relationships are called the second basic equations of molecular- kinetic theory gases They show that absolute temperature is the quantity that determines the average kinetic energy of the translational motion of molecules; it is a measure of the energy of translational motion of molecules, and thereby the intensity thermal movement molecules. This is the molecular kinetic meaning of absolute temperature. As we see, the process of heating a body is directly related to an increase in the average kinetic energy of the particles of the body. From (2.9) it is clear that absolute temperature is a positive quantity: 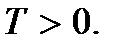 Meaning
Meaning 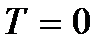 called absolute zero temperature. According to (2.8), at absolute zero the translational motion of particles should completely stop (
called absolute zero temperature. According to (2.8), at absolute zero the translational motion of particles should completely stop ( 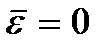 ). It should be noted, however, that at low temperatures the gas becomes condensed. Consequently, all conclusions drawn on the basis of the kinetic theory of gases lose their meaning. And at absolute zero temperature, motion does not disappear. The movement of electrons in atoms and the movement of free electrons in metals are completely preserved even at absolute zero temperature. In addition, even at absolute zero, some vibrational motion of atoms inside molecules and atoms at the nodes of a crystal lattice is preserved. The existence of these oscillations is associated with the presence of zero-point energy in the quantum harmonic oscillator (
). It should be noted, however, that at low temperatures the gas becomes condensed. Consequently, all conclusions drawn on the basis of the kinetic theory of gases lose their meaning. And at absolute zero temperature, motion does not disappear. The movement of electrons in atoms and the movement of free electrons in metals are completely preserved even at absolute zero temperature. In addition, even at absolute zero, some vibrational motion of atoms inside molecules and atoms at the nodes of a crystal lattice is preserved. The existence of these oscillations is associated with the presence of zero-point energy in the quantum harmonic oscillator ( 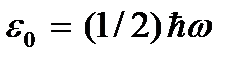 ), which can be considered the above vibrations of atoms. This energy does not depend on temperature, which means it does not vanish even at
), which can be considered the above vibrations of atoms. This energy does not depend on temperature, which means it does not vanish even at 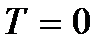 . At low temperatures, classical ideas about motion cease to hold true. In this area, quantum laws operate, according to which the movement of particles does not stop, even if the temperature of the body is reduced to absolute zero. But the speed of this movement no longer depends on temperature and this movement is not thermal. This is confirmed by the uncertainty principle. If the particles of the body were at rest, then their positions (coordinates x, y, z) and impulses (projections of impulse p x, p y, p z) would be precisely determined
. At low temperatures, classical ideas about motion cease to hold true. In this area, quantum laws operate, according to which the movement of particles does not stop, even if the temperature of the body is reduced to absolute zero. But the speed of this movement no longer depends on temperature and this movement is not thermal. This is confirmed by the uncertainty principle. If the particles of the body were at rest, then their positions (coordinates x, y, z) and impulses (projections of impulse p x, p y, p z) would be precisely determined 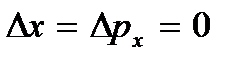 etc., and this contradicts the uncertainty relations
etc., and this contradicts the uncertainty relations 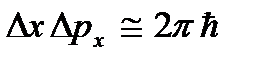 etc. Absolute zero is not achievable. It will be shown below that absolute zero temperature means a state of the system in which the system is in a state with the lowest energy, and therefore a further decrease in the intensity of the movement of its particles due to the transfer of its energy to surrounding bodies is not possible.
etc. Absolute zero is not achievable. It will be shown below that absolute zero temperature means a state of the system in which the system is in a state with the lowest energy, and therefore a further decrease in the intensity of the movement of its particles due to the transfer of its energy to surrounding bodies is not possible.
Formula (2.7) can be written in the form.
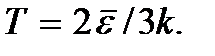
This formula can serve as a definition of the concept of absolute temperature for a monatomic gas. The temperature of any other system can be defined as a value equal to the temperature of a monatomic gas in thermal equilibrium with this system. The determination of temperature using this formula is correct up to temperatures at which the probability of the occurrence of electronically excited states of gas atoms can no longer be neglected.
Relation (2.8) allows us to introduce the so-called root mean square velocity of a molecule, defining it as
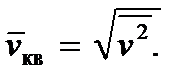
Then we get
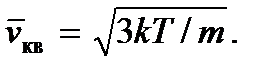
The concept of absolute temperature can be more strictly introduced in statistical physics, where it can be considered as the modulus of the statistical distribution of particles by energy. Note also that since temperature, like pressure, as can be seen from formulas (2.7) and (2.8), is determined by the average kinetic energy of an ideal gas molecule, then they represent statistical quantities and, therefore, it makes no sense to talk about the temperature or pressure of one or a small number of molecules.
