4.3.1. General information
about atomic nuclei. Isotopes
The composition of atomic nuclei includes two types elementary particles- protons and neutrons. A proton has a positive charge, equal in magnitude to the charge of an electron, and a rest mass m p = 1.6726·10 -27 kg. The neutron has no charge, its mass is slightly greater than the mass of the proton: m n = 1.6749·10 -27 kg. The common name for these particles is nucleons.
Charge atomic nucleus any chemical element, expressed in elementary charges, is equal to the atomic number Z of this element in D. Mendeleev’s Periodic Table. The charge of the nucleus is composed of the charges of protons, therefore, the number of protons in the atomic nucleus is equal to the atomic number of the element.
Almost all the mass of an atom is concentrated in its nucleus. Therefore, the sum of the numbers of protons and neutrons must be equal to the mass number of the atom:
| (4.3.1) |
The number of neutrons in the nucleus is equal to the difference between the mass number and the atomic number of the element.
Atoms whose nuclei have the same number of protons but different numbers of neutrons are called isotopes. All isotopes of one chemical element have the same structure of electron shells and, therefore, the same chemical properties.
The stability of the atomic nuclei of most elements suggests that the nuclear forces are exceptionally strong: they must exceed the significant Coulomb electrostatic repulsion forces that exist between protons in the nucleus. Nuclear forces appear only at very small distances of 10 -13 cm. With a slight increase in the distance between nucleons, nuclear forces decrease to zero, and Coulomb forces destroy the nucleus.
Nuclear forces are forces of a special kind, differing in nature from electrical and gravitational ones.
The most stable nuclei of light elements are those consisting of approximately the same number neutrons and protons. The heaviest elements (located in the Periodic Table after bismuth), whose nuclei consist of large number nucleons with a predominance of neutrons, nuclear forces no longer ensure the stability of the nucleus. Such nuclei spontaneously decay, turning into nuclei of lighter elements. This phenomenon is called natural radioactivity.
4.3.2. Natural radioactivity.
Alpha, beta, gamma radiation.
Natural radioactivity was discovered by Henri Becquerel in 1896 in uranium salts. It was found that invisible rays cause luminescence, ionize gases, and penetrate through opaque barriers, exposing photographic plates. Natural radioactivity is characteristic not only of uranium, but also of many other heavy elements - actinium, polonium, radium, thorium, etc. Such elements were called radioactive.
Radioactive radiation consists of three various types: alpha, beta, gamma radiation.
Alpha rays are deflected by electric and magnetic fields (Fig. 4.3.1) and represent a stream of helium atomic nuclei (alpha particles).
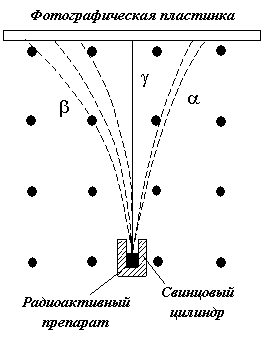
Rice. 4.3.1. Influence magnetic field(directed perpendicular
plane of the drawing towards the observer) for radioactive radiation
Each alpha particle has a charge of +2e and has a mass number of 4. Alpha particles are emitted from the nuclei of radioactive elements at speeds from 14,000 to 20,000 km/s, which corresponds to energies from 4 to 9 MeV.
Flying through matter, an alpha particle ionizes its atoms, acting on them with its electric field, i.e. knocks electrons out of the atoms of a substance. Having spent energy on ionization, the alpha particle slows down and captures two electrons from among those free in the substance, turning into an atom of helium gas. The path traveled by an alpha particle in a substance (before stopping) is called its range or penetrating power, and the number of ion pairs created during the journey is called its ionizing power. The greater the ionizing ability, the shorter the range of the particle in the substance.
The range of alpha particles in the air under normal conditions is 3-9 cm, and their ionizing ability is 100,000-250,000 ion pairs (an average of 30,000 ion pairs per 1 cm of range). Alpha particles have high ionizing ability and low penetrating ability.
Alpha rays are completely absorbed by a 0.06 cm thick layer of aluminum or a 0.12 cm thick layer of biological tissue.
Beta rays are deflected by electric and magnetic fields; represent a stream of fast electrons and are called β-particles. Their mass is 7360 times less than the mass of an α particle. The average speed of beta particles is about 160,000 km/s. It follows that β-particles are deflected by the magnetic field in the direction opposite to the deflection of α-particles, which is explained by the opposite charge.
Unlike alpha rays, beta rays contain particles with all possible energies (all possible speeds). Nuclei of the same radioactive element emit β-particles at both a speed close to zero and a speed close to the speed of light. The energy of β-particles ranges from hundredths to several MeV.
Since the β particle has a very small mass, high speed, and its charge is half that of the α particle, its ionizing ability is approximately 100 times less, and its range is the same number of times greater than that of the α particle. The range of a high-energy β-particle reaches 40 cm in air, 2 cm in aluminum, and 6 cm in biological tissue.
Gamma rays represent a stream of photons that have very high frequency, about 1020 Hz, which corresponds to a wavelength of the order of 10 -12 m. The energy of γ quanta is about 1 MeV.
Being hard electromagnetic radiation, γ-rays are similar in their properties to characteristic x-ray radiation. They are not deflected by electric and magnetic fields, propagate at the speed of light, and experience diffraction when passing through crystals. Unlike X-rays, gamma rays are emitted by the atomic nucleus.
Ionizing power is low; in air it has about 100 pairs of ions (on average 1-2 pairs of ions per 1 cm of travel). γ-rays are one of the most penetrating radiations. The hardest γ-rays pass through a 5 cm layer of lead or through a layer of air several hundred meters thick; penetrate through the human body.
4.3.3. Laws of alpha and beta decay
Radioactive radiation occurs as a result of the decay of radioactive elements. Obviously, the atoms of the radiating element must be transformed into atoms of another chemical element.
When a β-particle is emitted, the nuclear charge increases by one, but the mass remains virtually unchanged. Therefore, as β decays, a radioactive element turns into another element with an atomic number one higher and with the same mass number.
During β-decay, an element shifts one number to the right in the Periodic Table without changing its mass number.
β-decay scheme:
For example,
When an alpha particle is emitted, the nuclear charge decreases by two units and the mass number decreases by 4 units. Hence,
During α-decay, an element shifts two numbers to the left in the periodic table with a decrease in mass number by four units:
For example,
Rules (4.3.2) and (4.3.4) are called displacement laws.
Radioactive decay leads to a gradual decrease in the number of atoms of a radioactive element. It is random in the sense that it is impossible to predict which atom will decay and when. We can only talk about the probability of such a collapse.
The number of atoms decaying over some time turned out to be proportional total number atoms and times:
where λ is the proportionality coefficient, called the decay constant of a given element. The minus sign indicates a decrease in the atoms of the radioactive element over time.
Integrating (4.3.6), we obtain:
where N 0 is the number of atoms of the element at the initial moment of time.
Relationship (4.3.7) is called the law of radioactive decay (Fig. 4.3.2).
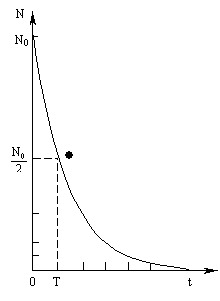
Rice. 4.3.2. Radioactive decay curve
To characterize the rate of decay, the concept of half-life T is introduced:
Half-life is the time during which the number of atoms of the original element is halved.
From (4.3.7) it follows that if e -λT = ½, then the following holds:
The reciprocal of the decay constant is called the average lifetime of a radioactive atom:
Consequently, Т = τln2, whence τ = Т/ ln2 = 1.44T, i.e. the average lifetime is approximately one and a half times longer than the half-life.
The half-life of uranium is 4.5 10 9 years, polonium is 1.5 10 -4 s.
The number of atomic decays occurring in a radioactive element in 1 s is called the activity of this element:
You can show what is running:
Thus, the activity of an element is proportional to its quantity and inversely proportional to its half-life. The unit of activity is taken to be the activity of 1 g of radium (1 Curie):
1 Ku = 3.7 10 10 dispersion/s.
The product of radioactive decay may itself be radioactive. Therefore, the process of radioactive decay goes through a number of intermediate stages, forming a chain of radioactive elements ending with a stable element. Such a chain of elements is called a radioactive family.
The unit of activity is Becquerel(Bk) this ; ![]()
The most commonly used unit of activity is the Curie (Ci)
Or mCi - millicurie 10 -3 Ci, mCi - micro curie 10 -6 Ci. There is also an extra-systemic Rutherford unit of activity (Rd) 1Rd = 10 6 Bq = 10 6 s -1. To characterize the activity of a unit mass of a radioactive source, a quantity called specific mass activity and equal to the ratio of the activity of the isotope to its mass. Specific mass activity is expressed in Becquerels per kilogram (Bq/kg) or Ci/kg, Ci/g or Ci/l.
4.3.4. β+ positron decay, electron capture and internal conversion
![]() , where ν is a neutrino particle, Q is the amount of heat. In this decay, the daughter element is shifted to the left by one cell in the periodic table.
, where ν is a neutrino particle, Q is the amount of heat. In this decay, the daughter element is shifted to the left by one cell in the periodic table.
A positron is a particle that has a charge like an electron, but positive
![]() .
.
For example, the decay of a phosphorus isotope:
![]()
In electron capture, one of the electrons is captured by the nucleus, with inner shell atom. As a result, the proton of the atom turns into a neutron.
![]()
A child element is shifted to the left in the periodic table
In electron capture, a proton turns into a neutron
![]() .
.
For example:
![]()
During decay, both α and β decays can occur
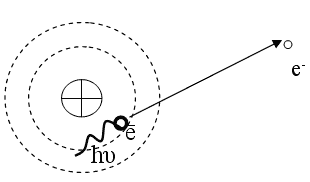
There are cases when the nucleus of atoms, being in an excited state, transfers part of its energy to electrons in the inner layers (K, L, M). As a result, the electron escapes from the atom. Such electrons are called internal conversion electrons. Consequently, the emission of conversion electrons is due to the direct electromagnetic interaction of the nucleus with shell electrons. Conversion electrons have a line energy spectrum, in contrast to beta decay electrons, which give a continuous spectrum. After internal conversion has occurred, a “vacant” place for the ejected conversion electron appears in the electron shell of the atom. One of the electrons from more distant layers (from higher energy levels) carries out a quantum transition to a “vacant” site with the emission of characteristic X-ray radiation.
4.3.5. Interaction of ionizing radiation with matter.
Charged particles and γ - photons, propagating in matter, interact with electrons and nuclei, as a result the state of matter and particles changes.
The main mechanism for the energy loss of charged particles (α and β) when passing through matter is ionization braking. The kinetic energy of particles is spent on excitation and ionization of atoms of the medium. This is quantified the following parameters: linear ionization density i, linear stopping power of matter S, average linear range.
Under linear density ionization i understand the ratio of the number dn of ions of the same sign formed by a charged ionizing particle along the elementary path dl: . Linear braking capacity substance S is the ratio of the energy dE lost by a charged ionizing particle when passing through an elementary path dl in a substance to the length of this path: . Average linear mileage of a charged ionizing particle R is the average distance between the beginning and end of the path of a charged particle in a given substance.
For α particles, the linear ionization density in air is ![]() , linear stopping power α of particles in air
, linear stopping power α of particles in air ![]() . The average linear path for α particles in the air is several cm, but in a living organism (10-100 microns), its path is rectilinear and changes the direction of movement only upon collisions with the nuclei of oncoming atoms.
. The average linear path for α particles in the air is several cm, but in a living organism (10-100 microns), its path is rectilinear and changes the direction of movement only upon collisions with the nuclei of oncoming atoms.
For β particles ![]() in the air, and the linear stopping power β of particles in the air
in the air, and the linear stopping power β of particles in the air ![]() . For β particles R, the average linear range in the air is 25 meters, and in a living organism up to 1 cm.
. For β particles R, the average linear range in the air is 25 meters, and in a living organism up to 1 cm.
In addition to ionization and excitation, β particles cause other processes:
1. Interacting with the electric field of the nucleus, the charged particle is decelerated and emits X-ray bremsstrahlung, the spectrum of which is shown in Fig. 4.3.3
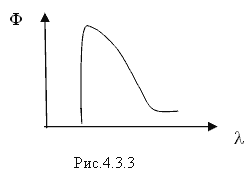
2. If an electron moves in a medium at a speed exceeding the speed of light in this medium, then characteristic Cherenkov radiation (Cherenkov-Vavilov radiation) appears.
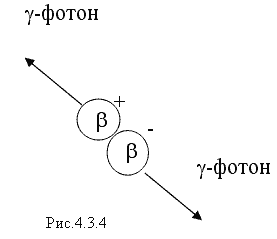
3. When a β + particle enters a substance, it is more likely that it interacts with electrons, resulting in the formation of two gamma photons instead of an electron-positron pair. This process, the diagram of which is shown in Fig. 4.3.4, is called annihilation. The energy of each γ - photon arising during annihilation must be no less than the rest energy of the electron or positron, i.e. not less than 0.51 MeV.
4.3.6. Interaction of gamma radiation with matter.
During radioactive decay, nuclei emit gamma rays with energies ranging from several keV to several MeV. Gamma rays, when passing through matter, lose energy practically due to three effects: photoelectric absorption (photoelectric effect), Compton scattering (Compton effect), and the formation of electron-positron pairs (pair formation). The magnitude of each effect depends on the atomic number of the absorbing material and the energy of the photon.
Photoelectric absorption.
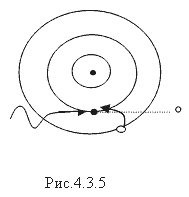
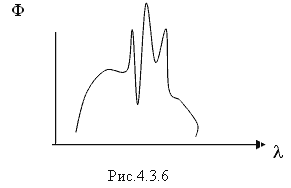
Fulfilled under the condition: hν³ A and, where A and is the work of ionization of an atom (the photoelectric effect diagram is shown in Fig. 4.3.5). The energy of a gamma quantum is calculated by the formula and does not exceed 50 keV. A gamma quantum, colliding with an electron more often than the K-layer in the atoms of the irradiated substance, completely transfers its energy, disappears itself, and the electron acquires kinetic energy equal to the energy of the gamma quantum minus the binding energy of the electron. in an atom. The electron jumps to the vacated space from the l - layer to the k - layer, the electron from the m - layer to the l layer, etc. During the transition, light quanta hν are emitted, creating characteristic X-ray radiation. The spectrum of characteristic X-ray radiation is shown in Fig. 4.3.6.
In air and biological tissues, the photoelectric effect is 50% if the energy of γ quanta is about 60 KeV. At Eγ=120 keV it is 10%, and starting from 200 keV this process is no longer observed. In this case, gamma radiation is attenuated due to Compton scattering.
Compton effect.
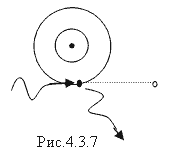
Fulfilled under the condition hν>>A and.
γ - quanta colliding with external valence electrons transfer only part of the energy. After colliding with them, γ - quanta change the direction of movement and are scattered. Electrons, breaking away from the nucleus, acquire significant kinetic energy and produce ionization of the substance (secondary ionization). The Compton effect diagram is shown in Fig. 4.3.7.
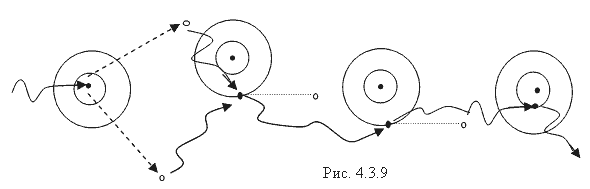
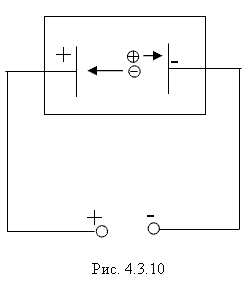
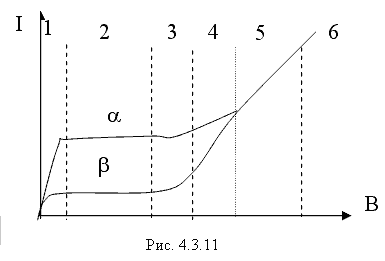
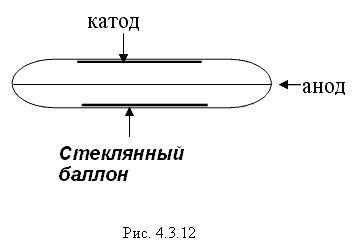
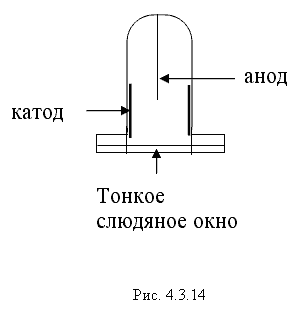
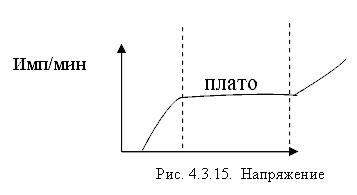
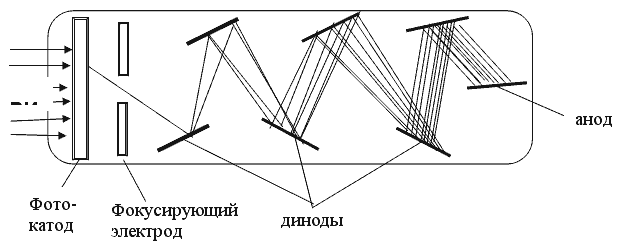
Due to the Compton effect, γ-radiation is weakened. This effect is significant in air and in biological matter at 200 KeV Energy balance for the Compton effect The kinetic energy of the electron, hν ’ is the newly formed scattered γ-gamma quantum of light. Thus, as a result of the Compton effect, the intensity of gamma radiation is weakened due to the fact that gamma quanta, interacting with electrons of the medium, are scattered in different directions and go beyond the primary beam, as well as due to the transfer of part of their energy to the electrons. Pair formation. Gamma quanta with energy E³ 1.02 MeV, passing through matter, are transformed under the influence of a strong electric field near the atomic nucleus into an electron-positron pair. In this case, there is a transformation of one form of matter - gamma radiation - into another - into particles of matter. The formation of such a pair of particles is possible only at γ-quanta energies no less than the energy equivalent to the mass of both particles - the electron and the positron. Since the masses of the electron and positron are the same, to form them without imparting additional kinetic energy to them, the energy of the γ-quantum must satisfy the relationship between mass and energy: If the energy of γ-quanta is more than 1.022 MeV, then its excess is transferred to particles. Then the kinetic energy of the resulting particles E k is equal to the difference between the photon energy Eγ and the double rest energy of the electron: The resulting electron-positron pair subsequently disappears (annihilates), turning into two secondary γ-quanta with an energy equal to the energy equivalent of the particle rest mass - 0.511 MeV. Secondary γ quanta can only cause the Compton effect and, ultimately, the photoelectric effect, i.e. lose energy only when colliding with electrons. The probability of the pair formation process increases with increasing γ-quanta energy and absorber density. The pair formation diagram is shown in Figure 4.3.8. High energy gamma rays (more than 8 MeV) can interact with atomic nuclei (nuclear effect). The probability of such an effect is very small, and this type of interaction practically does not weaken the radiation in the substance. The beam of gamma rays is absorbed continuously as the thickness of the absorber increases; its intensity does not vanish at any absorber thickness. This means that whatever the thickness of the layer of matter, it is impossible to completely absorb the flux of gamma rays, but can only weaken its intensity by any given number of times. In Fig. Figure 4.3.9 shows the dependence of the attenuation of gamma radiation on the thickness of the absorber. The mechanism of gamma radiation attenuation is shown in Fig. 10. Three types of gamma quantum scattering by an atom of a substance occur sequentially. First, the process of pair formation occurs, then Compton scattering and photoelectric absorption. During the last interaction with matter, the energy of the gamma quantum becomes less than the work of ionization of the atom and the weak gamma quantum encountering an atom of the substance is simply dissipated. The latter process is called coherent scattering. Law of beam attenuation of γ-rays has the following form I=I o e -μd, where I is the intensity of γ-rays transmitted through a substance of thickness d; I o - intensity of the incident beam of gamma rays; μ- linear attenuation coefficient. The linear attenuation coefficient is a total coefficient that takes into account the attenuation of the gamma ray beam due to the first three processes. Thus, μ = μ f + μ k + μ p. Since the value of μ depends on the energy of incoming gamma rays and on the absorber material, it can be expressed through the ratio μ/ρ, where ρ is the density of the substance. In this case, the coefficient μ will be called the mass attenuation coefficient and will no longer depend on the density of the material. The law of attenuation can be expressed in terms of half attenuation layer(Δ1/2). The thickness of the absorber, after passing through which the radiation intensity is attenuated by half, is called the half-attenuation layer Δ1/2, measured in units of surface density (mg/cm 2) and depends on the radiation energy and the density of the absorber. The relationship between the linear attenuation coefficient and the half-attenuation layer is as follows: Knowing the half-attenuation layer, you can quite easily determine what kind of absorber you need to take in order to attenuate the radiation by a given number of times. For example, one layer Δ1/2 reduces the radiation intensity by 2 times, two layers - by 4 times, three layers - by 8 times, etc., n layers - by 2 n times. Therefore, to attenuate the radiation, for example, by 512 times, you need to take so many layers Δ1/2n, so that 2 n =512. In our case n=9, i.e. 9 layers of half attenuation reduce the radiation intensity by 512 times. Radioactive radiation is not perceived by the senses. These radiations can be detected using special instruments. In practice, the most common are ionization radiation detectors that measure the direct effects of the interaction of radiation with matter - the ionization of a gaseous medium (ionization chambers, proportional counters and Geiger-Muller counters, as well as corona and spark counters). Other methods involve measuring secondary effects caused by ionization - photographic, luminescent, chemical, calorimetric, etc. Ionization radiation detectors - a chamber filled with air or gas with electrodes to create an electric field (Fig. 4.3.10). In the absence of U, there is no voltage between the electrodes in the current circuit, since gas is a good insulator. When charged (α, β) particles enter the gas, ion pairs are formed, and the gas becomes a conductor of the electric field. At the beginning, when U=0 at the electrodes, all the ions created by the initial ionization are completely recombined into neutral molecules. As the voltage increases, the ions acquire a directional effect: positive ones collect at the cathode, and negative ones at the anode. An ionization current occurs in the circuit, which can be recorded by the device. The magnitude of the ionization current serves as a measure of the amount of radiation. Figure 4.3.11 shows the dependence of the strength of the ionization current on the voltage applied to the detector electrodes. This dependence is called the current-voltage characteristic of the ionization detector. In section 1, there are two processes: the formation of charged particles-ions and the recombination of ions. As the voltage increases, the recombination process decreases, and all the resulting ions reach the electrodes - section 2. The magnitude of the current in section 2 depends only on the ionization ability of incoming charged particles. So α - a particle formed by a large ionizing effect, corresponds to the upper curve. Region 2 is called the ionization chamber region. In section 3, the strength of the ionization current begins to increase again, because positive ions, and especially negative ions, acquire significant acceleration and, consequently, energy in order to produce ionization themselves due to collisions with atoms or gas molecules. This process is called secondary ionization. In section 3, there is a strict proportionality between the number of primarily formed ions and the total amount of ions involved in the creation of the ionizing current. This region is called the proportionality region. Proportional counters operate in this mode. To do this, a gas gain coefficient Kg is introduced into the region - the ratio of the total sum of ions n participating in the creation of the ionization current to the number of primary ions n 0 formed. Kgy=n/ n 0. For section 3 Kgy reaches 10 3 - 10 4. In section 4, the strict proportionality between the number of primary formed ions and the strength of the ionization current is violated. Therefore, it is called the region of limited proportionality. In section 5, at even higher voltages, the strength of the increasing current no longer depends on the number of primarily formed ions. The gas amplification coefficient reaches 10 8 - 10 10 and when at least one nuclear particle appears in the detector chamber, a flash of an independent gas discharge occurs, which covers the entire chamber. This area is called the Geiger region. Counters operating in this area are called Geiger-Muller counters. In region 6, at high voltage, a constant continuous discharge is observed in the detector and the detector fails. 2. Proportional counters Proportional counters operate in section 3. The presence of proportional gain in the counters makes it possible to determine the energy of nuclear particles and study their nature. Typically, a proportional counter is made in the form of a cylinder, along the axis of which a metal thread, the anode, is pulled (Fig. 4.3.12). The conductive coating on the inner surface of the cylinder serves as the cathode. With such a device, the entire electric field is concentrated near the thread and its maximum value is higher, the smaller the radius of the thread (Fig. 4.3.13). Proportional counters are also manufactured in the end-face type (Fig. 4.3.14). To ensure penetration of alpha particles into the cavity of the counter, the entrance mica window is made very thin (4-10) µm. Fill the meter with a mixture of neon and argon almost to atmospheric pressure. There are open meters, the working cavity of which communicates with external air. Such meters operate at atmospheric pressure, they allow continuous flow or circulation of the gas filling them and therefore they are often used to record the activity of gas samples. Geiger-Muller (G-M) counters are structurally not much different from proportional counters of the cylindrical and end type. Its main difference is that the internal volume of the counter (G-M) is filled with an inert gas at reduced pressure, and the work is carried out in the Geiger region, i.e. in self-gas discharge mode. According to the principle of operation, meters (G-M) are divided into self-extinguishing and non-self-extinguishing. When a nuclear particle enters a non-self-quenching counter, primary ionization of the gaseous medium occurs. Positive ions move to the cathode and electrons move to the anode. In this case, under the influence of high voltage, electrons accelerate with great acceleration and produce secondary ionization. The newly formed ions also acquire a fairly high speed, produce ionization and knock electrons out of the cathode. These electrons further increase the avalanche effect. As a result, the entire counter is covered by the discharge. Kg can reach 10 8 - 10 10. If, during a rapidly increasing secondary ionization, the next nuclear particle penetrates into the non-self-quenching counter, it will not be registered by the counting unit. To detect a second nuclear particle, it is necessary to “extinguish” the ionization process from the first, which can be achieved either by including a high resistance in an electrical circuit or by introducing organic vapors into the counter. Such options are used in self-extinguishing meters. Typically, polyhydric alcohol vapors are used in a ratio of 90% argon and 10% alcohol vapors. The organic additive ensures the neutralization of positive argon ions by donating loosely bound electrons. Consequently, the molecules of the polyatomic gas (alcohol) stop secondary ionization, and the counter becomes ready to register the next particle. Dead time- this is the time during which the counter cannot register a particle (quantum) that has entered it. The dead time of self-extinguishing meters is 10 -4 s. Counter resolution- this is the maximum number of particles that the counter can register in one second and is calculated as the reciprocal of the dead time. The shorter the dead time, the greater the resolution of the counter. Non-self-quenching counters are capable of separately recording no more than 10 2 - 10 3 pulses/s, self-quenching ones - up to 10 4 pulses/s. Counter efficiency- this is the percentage ratio of the number of pulses registered by the counter to the total number of particles (quanta) that entered the working volume of the counter during the same period of time. Efficiency is determined by measuring the radiation of radioactive drugs with known activity (standard). Counting characteristics expresses the dependence of the counting speed (number of pulses/min) on the voltage applied to the counter. The voltage region in which constancy of the counting rate per unit time is established is called the “counter plateau”. The greater the length and the lower the slope of the plateau, the better the counter (Fig. 4.3.15). In self-extinguishing meters, the plateau length is 200-300 V, the slope is 3-5%. Scintillation (luminescent) method of recording radiation. When atoms transition from an excited state or from an ionized state to a ground state, energy is released in the form of a flash of light (scintillation), which can be detected, for example, by converting the light energy into an electrical signal using a photoelectric multiplier (PMT). The scintillation counter device diagram is shown in Figure 4.3.16. Under the influence of a light pulse generated in the scintillator, electrons are knocked out of the photocathode due to the photoelectric effect, which are collected by the electric field and directed to the first dynode, accelerating to an energy sufficient to knock out secondary electrons from the next dynode, etc. Thus, the electron avalanche increases from the cathode to the anode; very weak light flashes occurring in the scintillator are converted into recorded electrical impulses. Scintillation counters have a higher counting efficiency (up to 100%) and a resolution of 10 -5 when registering alpha particles and 10 -8 when registering beta particles, compared to gas-discharge counters. Semiconductor detectors(SPD) of ionizing radiation are a solid-state ionization chamber in which the role of electric charge carriers is performed by electrons and holes. Under the influence ionizing radiation An electric current is generated in the PPD. The magnitude of the current determines the amount of ionizing radiation. Photographic method is based on determining the degree of blackening of a photographic emulsion under the influence of ionizing radiation. The degree of blackening of the photographic plate emulsion is proportional to the radiation dose. Dosimetric photo monitoring (IFC) for persons working with beta and gamma radiation is based on this principle. Chemical methods are based on recording certain changes that occur under the influence of radiation. For example, color change, release of gases, precipitation of colloidal solutions, etc. The degree of change is proportional to the absorbed radiation energy. Ferrosulfate and cerium dosimeters, based on the oxidation of a divalent iron ion into a trivalent one under the influence of radiation, have become widespread. In a cerium dosimeter, the concentration of cerium is determined before and after irradiation. Calorimetric method is based on the measurement, using special calorimeters, of the thermal energy released when radiation energy is absorbed in a substance. Instruments for measuring ionizing radiation can be divided into three categories: radiometric (radiometers), dosimetric (dosimeters), units and devices of electronic equipment for nuclear physics research. Radiometers- these are devices with gas-discharge, scintillation counters and other detectors, designed to measure the activity of radioactive drugs and radiation sources, to determine the flux density or intensity of ionizing particles and quanta, surface radioactivity of objects, specific activity of aerosols, gases and liquids. Dosimeters (X-ray meters)
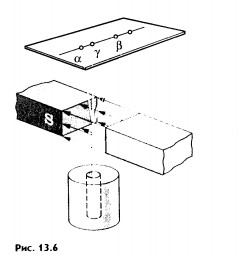
- devices measuring exposure and absorbed radiation doses or corresponding dose rates. Dosimeters consist of three main parts: a detector, a radio circuit that amplifies the ionization current, and a recording (measuring) device. Based on the principle of operation, dosimeters can be divided into two groups. The first group consists of dosimeters that measure the dose rate in roentgens per unit time, the so-called dose rate meters. The second group includes integrating dosimeters that measure the radiation dose over any period of time. The radiation detector in dose rate meters can be ionization chambers, a gas discharge or a scintillation counter. Ionization chambers are usually used as a detector in integrating devices. >> Alpha, beta and gamma radiation § 99 ALPHA, BETA AND GAMMA RADIATIONS After the discovery of radioactive elements, research began on the physical nature of their radiation. In addition to Becquerel and the Curies, Rutherford took up this task. The classic experiment that made it possible to detect the complex composition of radioactive radiation was as follows. The radium preparation was placed at the bottom of a narrow channel in a piece of lead. There was a photographic plate opposite the channel. The radiation emerging from the channel was affected by a strong magnetic field, the induction lines of which were perpendicular to the beam (Fig. 13.6). The entire installation was placed in a vacuum. In the absence of a magnetic field, one dark spot was detected on the photographic plate after development exactly opposite the channel. In a magnetic field, the beam split into three beams. The two components of the primary flow were deflected in opposite directions. This indicated that these radiations had electric charges opposite signs. In this case, the negative component of the radiation was deflected by the magnetic field much more strongly than the positive one. The third component was not deflected by the magnetic field at all. The positively charged component is called alpha rays, the negatively charged component is called beta rays, and the neutral component is called gamma rays (-rays, -rays, -rays). These three types of radiation differ greatly in penetrating power, that is, in how intensely they are absorbed various substances. -rays have the least penetrating ability. A layer of paper about 0.1 mm thick is already opaque for them. If you cover a hole in a lead plate with a piece of paper, then no spot corresponding to -radiation will be found on the photographic plate. Much less is absorbed when passing through matter - rays. The aluminum plate completely stops them only with a thickness of a few millimeters. .-rays have the greatest penetrating ability. The intensity of absorption of -rays increases with increasing atomic number of the absorbent substance. But a layer of lead 1 cm thick is not an insurmountable obstacle for them. When β-rays pass through such a layer of lead, their intensity weakens only by half. Physical nature-, - and -rays are obviously different. Gamma rays. In their properties, -rays are very similar to X-rays, but their penetrating power is much greater than that of X-rays. This suggested that the -rays were electromagnetic waves. All doubts about this disappeared after the diffraction of β-rays on crystals was discovered and their wavelength was measured. It turned out to be very small - from 10 -8 to 10 -11 cm. On the scale electromagnetic waves-rays directly follow X-rays. The speed of propagation of y-rays is the same as that of all electromagnetic waves - about 300,000 km/s. Beta rays. From the very beginning, - and - rays were considered as streams of charged particles. It was easiest to experiment with -rays, since they are more strongly deflected in both magnetic and electric fields. The main task of the experimenters was to determine the charge and mass of the particles. When studying the deflection of -particles in electric and magnetic fields, it was found that they are nothing more than electrons moving at speeds very close to the speed of light. It is important that the velocities of -particles emitted by any radioactive element are not the same. There are particles with very different speeds. This leads to the expansion of the beam of particles in a magnetic field (see Fig. 13.6). Alpha particles. It was more difficult to find out the nature of -particles, since they are weaker deflected by the magnetic and electric fields. Rutherford finally managed to solve this problem. He measured the ratio of a particle's charge q to its mass m by its deflection in a magnetic field. It turned out to be approximately 2 times less than that of a proton - the nucleus of a hydrogen atom. The charge of a proton is equal to the elementary one, and its mass is very close to the atomic mass unit 1. Consequently, the y-particle has a mass equal to two atomic mass units per elementary charge. But the charge of the particle and its mass remained, nevertheless, unknown. It was necessary to measure either the charge or the mass of the particle. With the advent of the Geiger counter, it became possible to measure charge more easily and accurately. Through a very thin window, particles can penetrate into the counter and be registered by it. Rutherford placed a Geiger counter in the path of the particles, which measured the number of particles emitted by a radioactive drug over a certain time. Then he replaced the counter with a metal cylinder connected to a sensitive electrometer (Fig. 13.7). Using an electrometer, Rutherford measured the charge - particles emitted by the source inside the cylinder in the same time (the radioactivity of many substances almost does not change with time). Knowing the total charge of the -particles and their number, Gezerfod determined the ratio of these quantities, i.e., the charge of one -particle. This charge turned out to be equal to two elementary ones. Thus, he established that the y-particle has two atomic mass units for each of the two elementary charges. Therefore, there are four atomic mass units per two elementary charges. The same charge and the same relative atomic mass has a helium nucleus. It follows from this that a particle is the nucleus of a helium atom. Not content with the achieved result, Rutherford then proved through direct experiments that it is helium that is formed during radioactive decay. Collecting -particles inside a special container for several days, he, using spectral analysis, was convinced that helium was accumulating in the vessel (each -particle captured two electrons and turned into a helium atom). During radioactive decay, -rays (nuclei of a helium atom), -rays (electrons) and -rays (short-wave electromagnetic radiation) are produced. Why did it turn out to be much more difficult to find out the nature of -rays than in the case of -rays? Myakishev G. Ya., Physics. 11th grade: educational. for general education institutions: basic and profile. levels / G. Ya. Myakishev, B. V. Bukhovtsev, V. M. Charugin; edited by V. I. Nikolaeva, N. A. Parfentieva. - 17th ed., revised. and additional - M.: Education, 2008. - 399 p.: ill. Planning physics lessons online, tasks and answers by class, homework 11th grade physics download It's no secret that radiation is harmful. Everyone knows this. Everyone has heard about the terrible casualties and the dangers of radioactive exposure. What is radiation? How does it arise? Are there different types radiation? And how to protect yourself from it? The word "radiation" comes from the Latin radius and denotes a ray. In principle, radiation is all types of radiation existing in nature - radio waves, visible light, ultraviolet and so on. But there are different types of radiation, some of them are useful, some are harmful. We are in ordinary life We are accustomed to using the word radiation to describe harmful radiation resulting from the radioactivity of certain types of substances. Let's look at how the phenomenon of radioactivity is explained in physics lessons. We know that atoms of matter consist of a nucleus and electrons rotating around it. So the core is, in principle, a very stable formation that is difficult to destroy. However, the atomic nuclei of some substances are unstable and can emit into space different energy and particles. This radiation is called radioactive, and it includes several components, which are named according to the first three letters of the Greek alphabet: α-, β- and γ- radiation. (alpha, beta and gamma radiation). These radiations are different, and their effect on humans and measures to protect against it are also different. Let's look at everything in order. Alpha radiation is a stream of heavy, positively charged particles. Occurs as a result of the decay of atoms of heavy elements such as uranium, radium and thorium. In the air, alpha radiation travels no more than five centimeters and, as a rule, is completely blocked by a sheet of paper or the outer dead layer of skin. However, if a substance that emits alpha particles enters the body through food or air, it irradiates internal organs and becomes dangerous. Beta radiation is electrons that are much smaller than alpha particles and can penetrate several centimeters deep into the body. You can protect yourself from it with a thin sheet of metal, window glass, and even ordinary clothing. When beta radiation reaches unprotected areas of the body, it usually affects the upper layers of the skin. During an accident at Chernobyl nuclear power plant in 1986, firefighters suffered skin burns as a result of very high exposure to beta particles. If a substance that emits beta particles enters the body, it will irradiate internal tissues. Gamma radiation is photons, i.e. electromagnetic wave carrying energy. In the air it can travel long distances, gradually losing energy as a result of collisions with atoms of the medium. Intense gamma radiation, if not protected from it, can damage not only the skin, but also internal tissues. Dense and heavy materials such as iron and lead are excellent barriers to gamma radiation. As you can see, according to its characteristics, alpha radiation is practically not dangerous if you do not inhale its particles or eat them with food. Beta radiation can cause skin burns due to exposure. Gamma radiation has the most dangerous properties. It penetrates deep into the body, and it is very difficult to remove it from there, and the effects are very destructive. In any case, without special instruments, it is impossible to know what type of radiation is present in this particular case, especially since you can always accidentally inhale radiation particles in the air. That's why general rule one thing is to avoid such places, and if you do find yourself, then wrap yourself in as much clothing and things as possible, breathe through the fabric, do not eat or drink, and try to leave the place of infection as quickly as possible. And then, at the first opportunity, get rid of all these things and wash yourself thoroughly. Radioactivity can also be seen as evidence of the complex structure of atoms. Initially, ancient philosophers imagined the smallest particle of matter - an atom - indivisible particle. How did radioactivity destroy this idea? Details at the link. Radioactivity refers to the property of spontaneous emission of any substances in the absence of external influences. Radioactive properties were first discovered in uranium in 1896 by French physicist Henri Becquerel (experiment with uranium salts) Subsequently, it was found that all chemical elements with an atomic number greater than 83 are radioactive. Properties of radioactive radiation 1. Cause ionization of gases 2. Have a chemical effect 3. Radioactivity is not a molecular phenomenon, but an internal property of the atoms of a radioactive element 4. Radioactivity of the drug with any chemical composition equal to the radioactivity of pure radioactive elements taken in the quantity in which they are contained in this preparation 5. Radioactive radiation does not depend on external influences (heating, increased pressure), chemical reactions, into which radioactive substances enter do not affect the intensity of radiation. 6. As a result of radioactive radiation, a completely new type of substance is formed, completely different in its physical and chemical properties from the original one. The chain of radioactive transformations ends with the formation of a non-radioactive (stable) isotope. 7. For each radioactive substance there is a certain time interval during which the activity decreases by 2 times. This interval is called the half-life. Half-life T is the time during which half of the available number of radioactive atoms decays. – law of radioactive decay N 0 – number of radioactive atoms at the initial moment of time N – number of radioactive atoms at a finite point in time t – time T – half life 8. A distinction is made between natural radioactivity (radioactivity of elements found in nature) and artificial radioactivity (radioactivity of elements obtained during nuclear reactions). To detect the complex composition of radioactive radiation, the following experiment was carried out: a radioactive preparation was placed at the bottom of a narrow channel in a piece of lead. There was a photographic plate opposite the channel. At the exit from the channel, the radiation was affected by a strong magnetic field, the induction lines of which were perpendicular to the beam. The entire installation was placed in a vacuum. In the absence of a magnetic field, one dark spot was discovered on the photographic plate after development, exactly opposite the channel. In a magnetic field, the beam split into three beams. Alpha radiation This is a stream of positively charged particles - the nuclei of helium atoms. The speed of alpha particles is significantly lower than the speed of beta particles and lies in the range of 10,000-20,000 km/s. The kinetic energy of alpha particles is high: 4-10 MeV. Alpha radiation has the least penetrating power. A layer of paper about 0.1 mm thick completely stops them. Beta radiation This is a stream of fast electrons emitted from the atoms of a radioactive substance. The speeds of beta particles are enormous and amount to 0.99 times the speed of light. The energy of beta particles reaches several megaelectronvolts. Beta radiation is average in its penetrating power. They are held back by an aluminum plate several millimeters thick. Gamma radiation This is a stream of electromagnetic waves of very short length (10 -8 - 10 -11 cm). The speed of propagation of gamma rays in a vacuum is the same as that of other electromagnetic waves - 300,000 km/s. Gamma radiation has the greatest penetrating power. A layer of lead 1 cm thick reduces the intensity of gamma radiation by half. Gamma rays and x-rays equal length The waves, except for the method of production, are no different from each other.![]() , Where
, Where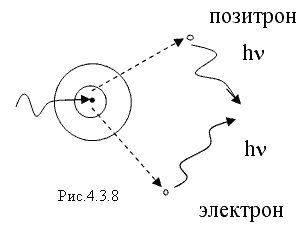
4.3.7. Law of attenuation of gamma radiation by matter
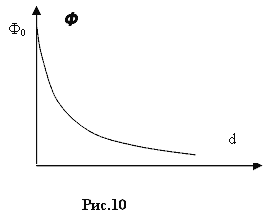
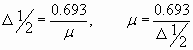 .
.4.3.8. Methods for detecting and recording ionizing radiation.
1. Ionization radiation detectors

3. Meter characteristics
Instruments for measuring radiation and their purpose.
1 Atomic mass unit (a.s.m.) rapia 1/12 the mass of a carbon atom; 1 a. e.m. 1.66057 10 -27 kg.Radioactivity in physics
Alpha radiation
Beta radiation
Gamma radiation
