In lesson 5 " Chemical formula" from the course " Chemistry for dummies» we will define chemical formulas and their indices, and also find out the differences between the chemical formulas of molecular and non-molecular structure. Let me remind you that in the last lesson “” we defined chemical compounds, looked at the differences between organic and inorganic compounds, and also found out what qualitative and quantitative composition means.
The composition of any substance is expressed as chemical formula.
Chemical formula is a conventional notation of the composition of a substance using chemical symbols and indices.
The qualitative composition is shown using the signs (symbols) of chemical elements, and the quantitative composition - using indices that are written to the right and just below the signs of the chemical elements.
Index- number of atoms of a given chemical element in the formula of a substance.
For example, the chemical formula of the simple substance hydrogen is written as follows:
and reads "ash-two".
Chemical formulas of substances of molecular structure
Formulas of diatomic molecules: oxygen - O 2 ("o-two"), chlorine - Cl 2 ("chlorine-two"), nitrogen - N 2 ("en-two"). The triatomic ozone molecule and the eight-atomic sulfur molecule are designated by the formulas O 3 (“o-three”) and S 8 (“es-eight”).
The formulas of molecules of complex substances also reflect their qualitative and quantitative composition. For example, the formula of water, as you probably already know well, is H 2 O (“ash-two-o”), methane is CH 4 (“tse-ash-four”), and ammonia is NH 3 (“en- ash-three"). The formulas of any complex substances are read in the same way. For example, the formula of sulfuric acid is H 2 SO 4 (“ash-two-es-o-four”), and that of glucose is C 6 H 12 O 6 (“tse-six-ash-twelve-o-six”).
Chemical formulas substances of molecular structure (they are called molecular formulas) show the composition of elementary parts, i.e., the conventional “bricks” of which these substances are composed. Such elementary components ( elementary structural units, or simply structural units) in this case are molecules.
What if the substance has a non-molecular structure? Chemical formulas simple substances of this type (for example, metals) are simply written with the signs of the corresponding elements without subscripts (or, more correctly, with a subscript, equal to one, which is not recorded). Thus, the formula of the simple substance of iron is Fe, copper is Cu, aluminum is Al.
The composition of complex substances of non-molecular structure is expressed using formulas that show simplest ratio of atomic numbers different chemical elements in these substances. Such formulas are called the simplest. For example, the simplest formula for quartz - the main component of river sand - is SiO 2. It shows that in a quartz crystal there are two oxygen atoms per silicon atom, i.e. the simplest ratio of the numbers of silicon and oxygen atoms in this substance is 1:2. The simplest formula Al 2 O 3 shows that in this compound the simplest ratio between the numbers of aluminum and oxygen atoms is 2:3.
A group of atoms whose composition corresponds to the simplest formula of a substance of non-molecular structure is called its formula unit.
The formula unit, table salt NaCl (“sodium chlorine”) is a group of one sodium atom and one chlorine atom. The chalk formula unit CaCO 3 (“calcium-ce-o-three”) is a group of one calcium atom, one carbon atom and three oxygen atoms.
The formulas of more complex compounds of non-molecular structure are read similarly. Additionally, only the number of groups of atoms enclosed in parentheses is indicated: Al 2 (SO 4) 3 (“aluminum-two-es-o-four-three times”), Mg(NO 3) 2 (“magnesium-en-o-three -twice"), etc.
Thus, structural units of substances molecular structure are molecules. Structural units of substances non-molecular structure are their formula units .
The table below shows the formula notation and schematic representation of the composition of substances of various types.
Brief conclusions of the lesson:
- The qualitative and quantitative composition of a substance is expressed using chemical formulas.
- The chemical formula of a substance with a molecular structure shows the composition of its molecule, which is the elementary structural unit of this substance.
- The chemical formula of a substance of non-molecular structure shows the simplest ratio of atoms in its formula unit.
Hope lesson 5" Chemical formula"was clear and informative. If you have any questions, write them in the comments.
Classification inorganic substances and their nomenclature is based on the simplest and most constant characteristic over time - chemical composition , which shows the atoms of the elements that form a given substance in their numerical ratio. If a substance is made up of one atom, i.e. is the form of existence of this element in free form, then it is called simple substance; if the substance is made up of atoms of two or more elements, then it is called complex substance. All simple substances (except monatomic ones) and all complex substances are usually called chemical compounds , since in them atoms of the same or different elements are connected to each other chemical bonds.
The nomenclature of inorganic substances consists of formulas and names. Chemical formula - depiction of the composition of a substance using symbols of chemical elements, numerical indices and some other signs. Chemical name - image of the composition of a substance using a word or group of words. The construction of chemical formulas and names is determined by the system nomenclature rules.
The symbols and names of chemical elements are given in the Periodic Table of Elements by D.I. Mendeleev. The elements are conventionally divided into metals And nonmetals . Non-metals include all elements of group VIIIA (noble gases) and group VIIA (halogens), elements of group VIA (except polonium), elements nitrogen, phosphorus, arsenic (VA group); carbon, silicon (IVA group); boron (IIIA group), as well as hydrogen. The remaining elements are classified as metals.
When compiling the names of substances, Russian names of elements are usually used, for example, dioxygen, xenon difluoride, potassium selenate. Traditionally, for some elements, the roots of their Latin names are introduced into derivative terms:
For example: carbonate, manganate, oxide, sulfide, silicate.
Titles simple substances consist of one word - the name of a chemical element with a numerical prefix, for example:
 The following are used numerical prefixes:
The following are used numerical prefixes:
 An indefinite number is indicated by a numeric prefix n- poly.
An indefinite number is indicated by a numeric prefix n- poly.
For some simple substances they also use special names such as O 3 - ozone, P 4 - white phosphorus.
Chemical formulas complex substances made up of the designation electropositive(conditional and real cations) and electronegative(conditional and real anions) components, for example, CuSO 4 (here Cu 2+ is a real cation, SO 4 2- is a real anion) and PCl 3 (here P +III is a conditional cation, Cl -I is a conditional anion).
Titles complex substances composed according to chemical formulas from right to left. They are made up of two words - the names of electronegative components (in the nominative case) and electropositive components (in the genitive case), for example:
CuSO 4 - copper(II) sulfate
PCl 3 - phosphorus trichloride
LaCl 3 - lanthanum(III) chloride
CO - carbon monoxide
The number of electropositive and electronegative components in the names is indicated by the numerical prefixes given above ( universal method), or oxidation states (if they can be determined by the formula) using Roman numerals in parentheses (the plus sign is omitted). In some cases, the charge of ions is given (for cations and anions of complex composition), using Arabic numerals with the corresponding sign.
The following special names are used for common multielement cations and anions:
For a small number of well-known substances it is also used special titles:
![]() 1. Acidic and basic hydroxides. Salts
1. Acidic and basic hydroxides. Salts
Hydroxides are a type of complex substances that contain atoms of some element E (except fluorine and oxygen) and hydroxyl groups OH; general formula of hydroxides E(OH) n, Where n= 1÷6. Form of hydroxides E(OH) n called ortho-shape; at n> 2 hydroxide can also be found in meta-form, which includes, in addition to E atoms and OH groups, oxygen atoms O, for example E(OH) 3 and EO(OH), E(OH) 4 and E(OH) 6 and EO 2 (OH) 2.
Hydroxides are divided into two groups with opposite chemical properties: acidic and basic hydroxides.
^ Acidic hydroxides contain hydrogen atoms, which can be replaced by metal atoms subject to the rule of stoichiometric valence. Most acid hydroxides are found in meta-form, and hydrogen atoms in the formulas of acidic hydroxides are given first place, for example, H 2 SO 4, HNO 3 and H 2 CO 3, and not SO 2 (OH) 2, NO 2 (OH) and CO (OH) 2. The general formula of acid hydroxides is H X EO at, where the electronegative component EO at X- called an acid residue. If not all hydrogen atoms are replaced by a metal, then they remain as part of the acid residue.
The names of common acid hydroxides consist of two words: the proper name with the ending “aya” and the group word “acid”. Here are the formulas and proper names of common acid hydroxides and their acidic residues (a dash means that the hydroxide is not known in free form or in an acidic aqueous solution):
Less common acid hydroxides are named according to nomenclature rules for complex compounds, for example:
 The names of acid residues are used to construct the names of salts.
The names of acid residues are used to construct the names of salts.
^ Basic hydroxides contain hydroxide ions, which can be replaced by acid residues subject to the rule of stoichiometric valence. All basic hydroxides are found in ortho-shape; their general formula is M(OH) n, Where n= 1.2 (less often 3.4) and M n+ - metal cation. Examples of formulas and names of basic hydroxides:
 The most important chemical property of basic and acidic hydroxides is their interaction with each other to form salts ( salt formation reaction), For example:
The most important chemical property of basic and acidic hydroxides is their interaction with each other to form salts ( salt formation reaction), For example:
Ca(OH) 2 + H 2 SO 4 = CaSO 4 + 2H 2 O
Ca(OH) 2 + 2H 2 SO 4 = Ca(HSO 4) 2 + 2H 2 O
2Ca(OH)2 + H2SO4 = Ca2SO4(OH)2 + 2H2O
Salts are a type of complex substances that contain M cations n+ and acidic residues*.
Salts with general formula M X(EO at) n called average salts, and salts with unsubstituted hydrogen atoms - sour salts. Sometimes salts also contain hydroxide and/or oxide ions; such salts are called main salts. Here are examples and names of salts:
 Acid and basic salts can be converted to middle salts by reaction with the appropriate basic and acidic hydroxide, for example:
Acid and basic salts can be converted to middle salts by reaction with the appropriate basic and acidic hydroxide, for example:
Ca(HSO 4) 2 + Ca(OH) = CaSO 4 + 2H 2 O
Ca 2 SO 4 (OH) 2 + H 2 SO 4 = Ca 2 SO 4 + 2H 2 O
There are also salts containing two different cations: they are often called double salts, For example:
 2. Acidic and basic oxides
2. Acidic and basic oxides
Oxides E X ABOUT at- products of complete dehydration of hydroxides:
 Acid hydroxides (H 2 SO 4, H 2 CO 3) acid oxides answer(SO 3, CO 2), and basic hydroxides (NaOH, Ca(OH) 2) - basic oxides(Na 2 O, CaO), and the oxidation state of element E does not change when moving from hydroxide to oxide. Example of formulas and names of oxides:
Acid hydroxides (H 2 SO 4, H 2 CO 3) acid oxides answer(SO 3, CO 2), and basic hydroxides (NaOH, Ca(OH) 2) - basic oxides(Na 2 O, CaO), and the oxidation state of element E does not change when moving from hydroxide to oxide. Example of formulas and names of oxides:
 Acidic and basic oxides retain the salt-forming properties of the corresponding hydroxides when interacting with hydroxides of opposite properties or with each other:
Acidic and basic oxides retain the salt-forming properties of the corresponding hydroxides when interacting with hydroxides of opposite properties or with each other:
N 2 O 5 + 2NaOH = 2NaNO 3 + H 2 O
3CaO + 2H 3 PO 4 = Ca 3 (PO 4) 2 + 3H 2 O
La 2 O 3 + 3SO 3 = La 2 (SO 4) 3
^ 3. Amphoteric oxides and hydroxides
Amphotericity hydroxides and oxides - a chemical property consisting in the formation of two rows of salts by them, for example, for aluminum hydroxide and aluminum oxide:
(a) 2Al(OH) 3 + 3SO 3 = Al 2 (SO 4) 3 + 3H 2 O
Al 2 O 3 + 3H 2 SO 4 = Al 2 (SO 4) 3 + 3H 2 O
(b) 2Al(OH) 3 + Na 2 O = 2NaAlO 2 + 3H 2 O
Al 2 O 3 + 2NaOH = 2NaAlO 2 + H 2 O
Thus, aluminum hydroxide and oxide in reactions (a) exhibit the properties main hydroxides and oxides, i.e. react with acidic hydroxides and oxide, forming the corresponding salt - aluminum sulfate Al 2 (SO 4) 3, while in reactions (b) they also exhibit the properties acidic hydroxides and oxides, i.e. react with basic hydroxide and oxide, forming a salt - sodium dioxoaluminate (III) NaAlO 2. In the first case, the element aluminum exhibits the property of a metal and is part of the electropositive component (Al 3+), in the second - the property of a non-metal and is part of the electronegative component of the salt formula (AlO 2 -).
If these reactions occur in an aqueous solution, then the composition of the resulting salts changes, but the presence of aluminum in the cation and anion remains:
2Al(OH) 3 + 3H 2 SO 4 = 2 (SO 4) 3
Al(OH) 3 + NaOH = Na
Here, complex ions 3+ - hexaaqualuminium(III) cation, - - tetrahydroxoaluminate(III) ion are highlighted in square brackets.
Elements that exhibit metallic and non-metallic properties in compounds are called amphoteric, these include elements of the A-groups of the Periodic Table - Be, Al, Ga, Ge, Sn, Pb, Sb, Bi, Po, etc., as well as most elements of the B- groups - Cr, Mn, Fe, Zn, Cd, Au, etc. Amphoteric oxides are called the same as basic ones, for example:
 Amphoteric hydroxides (if the oxidation state of the element exceeds + II) can be found in ortho- or (and) meta- form. Here are examples of amphoteric hydroxides:
Amphoteric hydroxides (if the oxidation state of the element exceeds + II) can be found in ortho- or (and) meta- form. Here are examples of amphoteric hydroxides:
 Amphoteric oxides do not always correspond to amphoteric hydroxides, since when trying to obtain the latter, hydrated oxides are formed, for example:
Amphoteric oxides do not always correspond to amphoteric hydroxides, since when trying to obtain the latter, hydrated oxides are formed, for example:
 If an amphoteric element in a compound has several oxidation states, then the amphotericity of the corresponding oxides and hydroxides (and, consequently, the amphotericity of the element itself) will be expressed differently. For low oxidation states, hydroxides and oxides have a predominance of basic properties, and the element itself has metallic properties, so it is almost always included in the composition of cations. For high degrees oxidation, on the contrary, in hydroxides and oxides there is a predominance of acidic properties, and the element itself has non-metallic properties, so it is almost always included in the composition of anions. Thus, manganese(II) oxide and hydroxide have dominant basic properties, and manganese itself is part of cations of the 2+ type, while manganese(VII) oxide and hydroxide have dominant acidic properties, and manganese itself is part of the MnO 4 - type anion. . Amphoteric hydroxides with a high predominance of acidic properties are assigned formulas and names modeled after acidic hydroxides, for example HMn VII O 4 - manganese acid.
If an amphoteric element in a compound has several oxidation states, then the amphotericity of the corresponding oxides and hydroxides (and, consequently, the amphotericity of the element itself) will be expressed differently. For low oxidation states, hydroxides and oxides have a predominance of basic properties, and the element itself has metallic properties, so it is almost always included in the composition of cations. For high degrees oxidation, on the contrary, in hydroxides and oxides there is a predominance of acidic properties, and the element itself has non-metallic properties, so it is almost always included in the composition of anions. Thus, manganese(II) oxide and hydroxide have dominant basic properties, and manganese itself is part of cations of the 2+ type, while manganese(VII) oxide and hydroxide have dominant acidic properties, and manganese itself is part of the MnO 4 - type anion. . Amphoteric hydroxides with a high predominance of acidic properties are assigned formulas and names modeled after acidic hydroxides, for example HMn VII O 4 - manganese acid.
Thus, the division of elements into metals and non-metals is conditional; Between the elements (Na, K, Ca, Ba, etc.) with purely metallic properties and the elements (F, O, N, Cl, S, C, etc.) with purely non-metallic properties, there is a large group of elements with amphoteric properties.
4. Binary compounds
A broad type of inorganic complex substances are binary compounds. These include, first of all, all two-element compounds (except for basic, acidic and amphoteric oxides), for example H 2 O, KBr, H 2 S, Cs 2 (S 2), N 2 O, NH 3, HN 3, CaC 2 , SiH 4 . The electropositive and electronegative components of the formulas of these compounds include individual atoms or bonded groups of atoms of the same element.
Multielement substances, in the formulas of which one of the components contains unrelated atoms of several elements, as well as single-element or multi-element groups of atoms (except hydroxides and salts), are considered as binary compounds, for example CSO, IO 2 F 3, SBrO 2 F, CrO (O2)2, PSI3, (CaTi)O3, (FeCu)S2, Hg(CN)2, (PF3)2O, VCl2 (NH2). Thus, CSO can be represented as a CS 2 compound in which one sulfur atom is replaced by an oxygen atom.
The names of binary compounds are constructed according to the usual nomenclature rules, for example:
 For some binary compounds, special names are used, a list of which was given earlier.
For some binary compounds, special names are used, a list of which was given earlier.
Chemical properties binary compounds are quite diverse, so they are often divided into groups by the name of anions, i.e. halides, chalcogenides, nitrides, carbides, hydrides, etc. are considered separately. Among binary compounds there are also those that have some characteristics of other types of inorganic substances. Thus, the compounds CO, NO, NO 2, and (Fe II Fe 2 III) O 4, the names of which are constructed using the word oxide, cannot be classified as oxides (acidic, basic, amphoteric). Carbon monoxide CO, nitrogen monoxide NO and nitrogen dioxide NO 2 do not have corresponding acid hydroxides (although these oxides are formed by non-metals C and N), nor do they form salts whose anions would include atoms C II, N II and N IV. Double oxide (Fe II Fe 2 III) O 4 - diiron(III)-iron(II) oxide, although it contains atoms of the amphoteric element - iron in the electropositive component, but in two different degrees oxidation, as a result of which, when interacting with acidic hydroxides, it forms not one, but two different salts.
Binary compounds such as AgF, KBr, Na 2 S, Ba(HS) 2, NaCN, NH 4 Cl, and Pb(N 3) 2 are built, like salts, from real cations and anions, which is why they are called salt-like binary compounds (or simply salts). They can be considered as products of the substitution of hydrogen atoms in the compounds HF, HCl, HBr, H 2 S, HCN and HN 3. The latter in an aqueous solution have an acidic function, and therefore their solutions are called acids, for example HF (aqua) - hydrofluoric acid, H 2 S (aqua) - hydrosulfide acid. However, they do not belong to the type of acid hydroxides, and their derivatives do not belong to the salts within the classification of inorganic substances.
CHEMISTRY TEST
14. What amount of Cr(OH) 3 contains the same number of equivalents as 174.96 g of Mg(OH) 2?
The molar mass of magnesium hydroxide is 58.32 g/mol, and the equivalent mass is 29.16 g/mol. Therefore, a mass of magnesium hydroxide of 174.96 g contains 174.96 g: 29.16 g/mol = 6 mol-equiv.
The molar mass of the equivalent of chromium hydroxide (III) is 1/3 M Cr(OH)3 or 34.34 g/mol, and 6 mol-equiv of this substance will be 34.33 g/mol x 6 mol = 205.98 g.
Thus, 205.98 g of Cr(OH)3 contains the same number of equivalents as 174.96 g of Mg(OH)2.
34. How many and what values can the magnetic quantum number m l take with the orbital quantum number l=0,1,2,3? What elements in the periodic table are called s-, p-, d-, f- elements? Give examples
The number of values of the magnetic quantum number depends on the orbital quantum number and is equal to
(21 + 1), where 1 is the orbital quantum number. Therefore, when l=0m l =0,
at l=1 m l takes values -1.0, +1;
at 1=2 m l takes values -2,-1, 0,+1,+2;
at =3m l can take values -3,-2,-1, 0, +1,+2,+3.
Chemical elements in the atoms of which the s-, p-, d-, f- orbitals are filled with electrons, respectively, are called s-, p-, d-, f-elements.
For example, s-elements include H, He, as well as alkali and alkaline earth metals (metals of 1A and 11A groups - Na, K, Rb, Be. Ca, Mg, Sg, etc.)
P-elements include, for example, elements that complete periods in the periodic system of elements (except for the first period) - B, C, N, Ne, J, Cl, Br, P, S, F, As, Se, Ar, Rn, Te and etc.
D-elements include elements located in large periods between s-elements and p-elements, for example, Fe, Mn, Cr, Ti, Mo, Pt, Co, Ru, Rh, etc.
The f-elements include lanthanides, for example, Ce, Nd, Pm, Sm, Eu, Gd, and actinides, for example, Th, U, Np, Pu, Am, etc.
54. What is the lowest oxidation state of hydrogen, fluorine, sulfur and nitrogen? Why? Make up formulas for calcium compounds with these elements in this oxidation state. What are the names of the corresponding compounds?
The hydrogen atom has a single valence electron. Therefore, the lowest oxidation state of hydrogen will be -1 (a hydrogen atom accepts 1 electron from another element). Hydrogen exhibits this lowest oxidation state +2 -1 in the compound CaH2. This compound is called calcium hydride.
The fluorine atom has seven valence electrons, one electron missing to complete the energy level. Therefore, the lowest (and only) oxidation state of fluorine is -1. Fluorine compounds in this +2 -1 oxidation state are called fluorides. For example, CaF 2 is calcium fluoride.
The sulfur atom has six valence electrons, two electrons are missing to complete the energy level. Therefore, the lowest oxidation state of sulfur is -2. Sulfur compounds in this oxidation state +2 -2 are called sulfides. For example, CaS is calcium sulfide.
The nitrogen atom has five valence electrons; three electrons are missing before the energy loss is complete. Therefore, the lowest oxidation state of nitrogen is -3. Nitrogen compounds in this oxidation state +2 -3 are called nitrides. Ca 3 N 2 is calcium nitride.
74. What should be understood by the oxidation state of an atom? Determine the oxidation state of the carbon atom and its valency in the compounds: CH 4 ; CH 3 OH; UNSC; CO 2
The oxidation state is the conditional charge of an atom in a compound, calculated from the assumption that it consists only of ions. The oxidation number can be negative, positive or zero, represent an integer or a fractional number. The algebraic sum of the oxidation states of atoms in a compound is always equal to zero, and in a complex ion it is equal to the charge of the ion.
In the given carbon compounds, the carbon atom is tetravalent. But the degree of oxidation of the carbon atom in these compounds is different.
In methane - CH 4 - the oxidation state of carbon is 4.
In methanol - CH3OH - carbon oxidation state - 2; in formic acid HCOOH - carbon oxidation state +2; in carbon dioxide - CO 2 - carbon oxidation state +4.
94. When burning 11.5 g of liquid ethyl alcohol 308.71 kJ of heat was released. Write the thermochemical equation for the reaction that results in the formation of water vapor and carbon dioxide. Calculate the heat of formation of C 2 H 5 OH (l). Answer: -277.67 kJ
C 2 H 5 OH (l) + 3 O 2 (g) = 2 CO 2 (g) + 3 H 2 O (p)
1 mole of liquid ethyl alcohol has a mass of 46 g.
To compile a thermochemical equation, let’s create the proportion:
11.5 g ethanol ----------- 308.71 kJ
46 g ethanol ------------ x kJ
Then the thermochemical equation of methane will take the form:
C 2 H 5 OH (l) + 3 0 2 (g) = 2 C0 2 (g) + 3 H 2 O (p); ∆Н° = -1234.84
We write the thermal effect of the reaction with a minus sign, since heat is released during the reaction.
According to Hess's law, the thermal effect of a reaction does not depend on the transition path, but depends only on the final and initial state of the system.
∆Н° reaction = ∑∆Н° final. - ∑∆Н° return start
We take the standard enthalpies of formation of the starting substances and reaction products from the reference book:
∆H° sample CO 2 (g) = -393.51 kJ/mol,
∆Н° arr H 2 O (steam) = -241.83 kJ/mol
∆H° sample O 2 (g) = 0 kJ/mol.
∆H° arr (C 2 H 5 OH (l)) = [∆H° arr (H 2 O) *3 + ∆H° arr (CO 2) *2] - ∆H° reaction = [(-241, 83)*3 + (- 393.51)*2 - (-1234, 84) = - 277.68 kJ/mol
114. Which of the carbonates: BeCO3, CaCO3 or BaCO3 can be obtained by the action of the corresponding oxides with CO 2? Which reaction occurs most energetically? Draw a conclusion by calculating ∆G° 298 reactions
Let's take from the reference book the standard values of the Gibbs energy ∆G° 298 for the starting substances and final products of reactions: ∆G° 298 BeO = -569.54 kJ/mol, ∆G° 298 BeCO3 = -944.75, ∆G° 298 BaO = -525.84 kJ/mol, ∆G° 298 ВаСО 3 = -1132.77 kJ/mol; ∆G° 298 CaO = -603.46 kJ/mol,
∆G° 298 CaCO 3 = -1128.35 kJ/mol; ∆G° 298 CO 2 = -394.37 kJ/mol;
Then for the reaction BeO(k) + CO 2 (g) = BeCO3(k) the standard value of the Gibbs energy will be:
944.75-[(-569.54)+(-394.37)]=+19.16 kJ/mol.
The positive value of the Gibbs energy for this reaction indicates that under standard conditions this reaction proceeds predominantly from right to left and beryllium cabonate cannot be obtained from BeO and CO 2.
For the reaction BaO(k) + CO 2 (g) = BaCO3(k) the standard value of the Gibbs energy will be:
1132.77-[(-525.84)+(-394.37)]=-212.56 kJ/mol/
For the reaction CaO(k) + CO 2 (g) = CaCO3(k) the standard value of the Gibbs energy will be:
1128.35-[(-603.46)+(-394.37)]=-130.52 kJ/mol.
The Gibbs energy value for these reactions is negative and these processes proceed in the forward direction, that is, calcium and barium carbonates can be obtained in this way. The most energetic reaction will occur between barium oxide and carbon dioxide, since the Gibbs energy value for this reaction will have the most negative value.
134. The equilibrium of the homogeneous system 4 HCl (g) + O 2 (g) ↔ 2H 2 0 (g) + 2Cl 2 (g) was established at the following concentrations of reactants mol/l: [H 2 O] p = 0.14; [Cl 2 ] p =0.14; [HCl] P = 0.20; [O 2 ] p = 0.32. Calculate the initial concentrations of hydrogen chloride and oxygen
[H 2 0] p = 0.14 mol/l
[Cl 2 ] p =0.14 mol/l
[HCl] P = 0.20 mol/l
[O 2 ] p = 0.32 mol/l
Ref =? ref=?
Based on the reaction equation, all chlorine (2 mol) is formed from hydrogen chloride (4 mol), and all water is formed from the original hydrogen chloride and oxygen.
Therefore, in order to form 0.14 mol of chlorine, 0.28 mol of hydrogen chloride must react, and in order to obtain 0.14 mol of water, 0.07 mol of oxygen must react.
Thus, the initial concentration of HCl was 0.20 + 0.28 = 0.48 mol/l, and the initial concentration of O 2 was equal to 0.32 + 0.07 = 0.39 mol/l.
Answer: The initial concentrations of hydrogen chloride and oxygen are 0.48 mol/L and 0.39 mol/L, respectively.
154. To neutralize 1 liter of solution containing 1.4 g of KOH, 50 cm 3 of acid solution is required. Calculate the molar concentration of the acid solution equivalent
Let's find the titer of the KOH solution:
Now let's calculate the molar concentration of the equivalent of the KOH solution:
Knowing the molar concentration of the equivalent of the KOH solution, we calculate the molar concentration of the acid equivalent:
174. How many grams of urea CO(NH 2) 2 should be dissolved in 75 g of water so that the crystallization temperature of the solution decreases by 0.465°? The cryoscopic constant of water is 1.86
The molar mass of urea is 60 g/mol. Decrease in the freezing temperature of the solution ∆T K = 0.465 °C.
According to Raoult's law, the decrease in the crystallization temperature of a solution compared to the crystallization temperature of a pure solvent depends on the cryoscopic constant of the solvent and the molal concentration of the solute. From here you can calculate the mass of the dissolved substance - urea in solution.
194. Write molecular and ion-molecular equations for reactions that are expressed by ion-molecular equations
Fe(OH)3 + 3 H + = Fe 3+ + 3 H 2 O
Cd 2+ + 2 OH - = Cd(OH) 2
H + + NO 2 - =HNO 2
Fe(OH) 3 (t)+ 3 HCl = FeCl 3 + 3 H 2 O
Fe(OH) 3 (t) + 3 H + + 3 Cl - = Fe 3+ + 3Cl - + 3 H 2 O
Fe(OH) 3 (t) + 3 H + = Fe 3+ + 3 H 2 O
Cd(NO 3) 2 + 2 KOH = Cd(OH) 2 (t)+ 2 KNO 3
Cd 2+ + 2NO 3 +2K + +2OH - = Cd(OH) 2 (t)+ 2 K + + 2 NO 3 -
Cd 2 + 2 OH - = Cd(OH) 2 (t)
HC1 + NaNO2 = НNO2 + NaС1
Н + + Сl - + Na + + NO 2 - = НNO 2 + Na + + Сl -
H + +NO 2 - =HNO 2
214. When mixing A1 2 (SO 4) 3 and Na 2 CO 3, each of the salts taken is hydrolyzed irreversibly to form the corresponding base and acid. Express this joint hydrolysis using ionic and molecular equations
A1 2 (SO 4) 3 + 3 Na 2 CO 3 + 3 H 2 O → 2 A1 (OH) 3 (t) + 3 Na 2 SO 4 + 3SO 2 (g)
2 A1 3+ + 3 SO 4 2- + 6 Na + + 3SO 3 2- + 3 H 2 O → 2 Fe(OH) 3 (t) + 6 Na + + 3 SO 4 2- + 3SO 2 (g)
2 A1 3+ + 3SO 3 2- + 3 H 2 O → 2 A1(OH) 3 (t) + 3SO 2 (g)
254. Iron and silver plates are connected by an external conductor and immersed in a solution of sulfuric acid. Draw a diagram of this galvanic cell and write electronic equations for the processes at the anode and cathode.
(-)Fe |H 2 S0 4 | |Н 2 S0 4 |Аg(+)
The process Fe-2 e = Fe 2+ occurs at the anode (iron), electrons pass through the conductor to the silver plate and the process occurs on the surface of the silver cathode
2 N + + 2 e = N 2 T.
The iron plate will dissolve and the silver plate will exhibit hydrogen evolution.
274. Compose electronic equations for the processes occurring on graphite electrodes during the electrolysis of a KBr solution. What mass of substance is released at the cathode and anode if electrolysis is carried out for 1 hour 35 minutes at a current of 15 A? Answer: 0.886g; 70.79g
With electrolysis aqueous solution potassium bromide with inert (graphite) electrodes at the cathode, the process of reduction of water molecules occurs and hydrogen is released:
2Н 2 О+2е =Н 2 +2О1H -
At the anode, the process of oxidation of bromide ions occurs and bromine is released: 2Br - - 2е = Br 2 The molar mass of hydrogen equivalent is 1 g/mol, and molar mass bromine equivalent is 79.904 g/mol.
Then, according to Faraday’s laws, one can find the mass of hydrogen and bromine, which are released at the cathode and anode, respectively.
294. Which metal is more appropriate to choose for tread protection against corrosion of the lead cable sheath: zinc, magnesium or chromium? Why? Compose electronic equations for the anodic and cathodic processes of atmospheric corrosion. What is the composition of corrosion products?
In sacrificial protection, the protector, a more active metal than the metal of the structure being protected, serves as an anode and is destroyed, thereby protecting the structure from destruction. Therefore, the more negative the potential of the protector metal, the more effective the protector protection will be. The lowest potential will be for magnesium -2.37 V, (for zinc - 0.763 V; for chromium - 0.74 V)
Since the cable sheath is made of lead, the magnesium-lead galvanic pair will have the greatest potential difference. And magnesium will serve as the best protective protection.
During atmospheric corrosion on the surface of a magnesium protector, the following process occurs:
2Mg – 4е = 2Mg 2+
Electrons pass through the conductor to the lead cable and oxygen depolarization occurs on the surface of the lead:
O 2 + 2 H 2 O + 4е = 4 OH -
The product of atmospheric corrosion will be magnesium hydroxide
2 Mg 2+ + 4 OH - = 2 Mg(OH) 2
314. Write expressions for the instability constants of the following complex ions: [Аg(СN) 2 ] - ; [Аg(NН 3) 2 ] + ; [Аg(SСМ) 2 ] - . Knowing that they are respectively equal to 1.0*10 -21, 6.8*10 -8, 2.1*10 -11, indicate in which solution containing these ions, with an equal molar concentration of Ag + ions, is there more?
Let us write down the expressions for the instability constant for these complex ions:
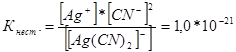
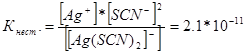
At a given temperature, the value of the instability constant is a constant value. The lower the instability constant, the more stable the complex, therefore the highest concentration of silver ions will be in a solution containing the complex ion [Аg(NНз) 2 ] +.
Literature
1. Akhmetov N.S. General and inorganic chemistry. M, 2002.
2. Karapetyants M.Kh., Drakin S.I. General and inorganic chemistry. M. 1994.
3. Glinka L. I. general chemistry. M. 1984.
