Mass defect. According to relativistic mechanics, the rest mass M stable system of interconnected particles is less than the sum of the rest masses m 1 + m 2 +…+ m k of the same particles taken in a free state. Difference
Δ M =(m 1 + m 2 +…+ m k) – M is called the mass defect of the particle system.
A decrease in the rest mass of free particles when they are combined into a stable system occurs due to the release of some part of the rest energy of these particles. The energy released is called binding energy.
From the law of conservation of energy it follows that the least energy that must be expended to divide a stable system of interconnected particles into individual free particles is equal to the binding energy.
The binding energy is directly proportional to the mass defect of the particle system Δ E = With 2Δ M, Where With 2 – coefficient of transition from mass to energy, numerically equal to the square of the speed of light in vacuum; .
If energy is expressed in megaelectron volts, and mass in atomic units, then With 2 = 931.44 MeV/amu
Mass defect Δ M atomic nucleus is the difference between the sum of the masses of free protons and neutrons and the mass of the nucleus formed from them Δ M = (Ζ m p+ Nm n) – M, Where Ζ – number of protons in the nucleus; N– number of neutrons ( N = A –Ζ); m p and m n – masses of free proton and neutron; M– core mass.
Nuclear reactions
Symbolic notation nuclear reaction can be given or in expanded form, for example 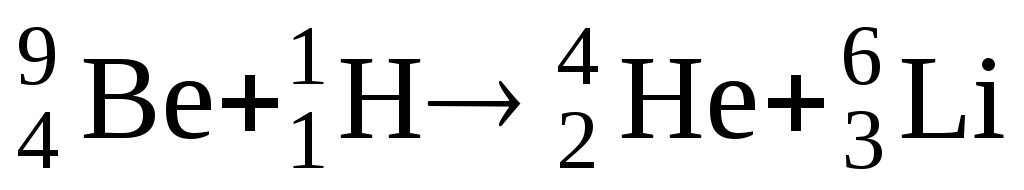 , or abbreviated 9 Be( R,α) 6 Li.
, or abbreviated 9 Be( R,α) 6 Li.
Particle designations: p – proton, n– neutron, d – deuteron, t– triton, α – alpha particle, γ – gamma photon.
When solving problems, conservation laws are applied:
number of nucleons A 1 + A 2 = A 3 +A 4 ;
charge Ζ 1 + Ζ 2 = Ζ 3 + Ζ 4;
relativistic total energy
E 1 + E 2 = E 3 + E 4 ;
impulse 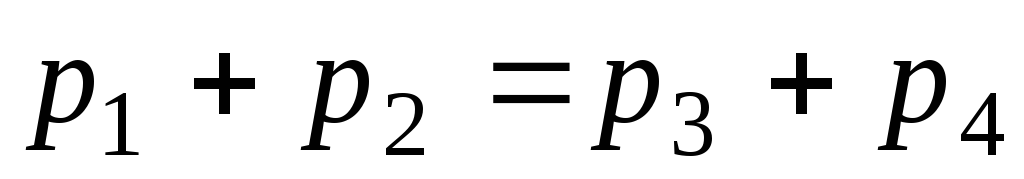 .
.
Energy effect of nuclear reaction Q = c 2 [(m 1 + m 2) – (m 3 +m 4)],
Where m 1 – rest mass of the target nucleus; m 2 – rest mass of the bombarding particle; m 3 + m 4 is the sum of the rest masses of the nuclei of the reaction products.
If m 1 + m 2 > m 3 +m 4, then energy is released, the energy effect is positive, the reaction is exothermic.
If m 1 + m 2 < m 3 +m 4, then energy is absorbed, the energy effect is negative, the reaction is endothermic.
4.1. Examples of problem solving
№ 1. The electron in the hydrogen atom has moved from the fourth energy level to the second. Determine the energy of the emitted photon.
Solution.
To determine the photon energy, we use the serial formula for hydrogen-like ions:
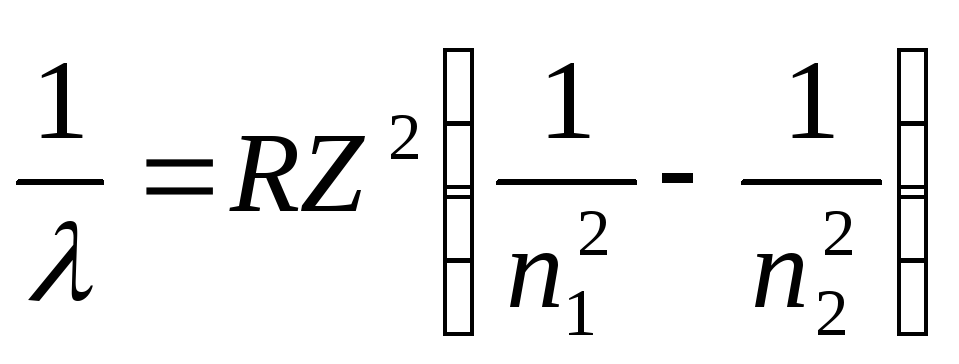 ,
(1)
,
(1)
Where - photon wavelength; R– Rydberg constant; Z– nuclear charge in relative units (at Z= 1 formula goes into the serial formula for hydrogen); n 1 – number of the orbit to which the electron moved; n 2 – number of the orbit from which the electron moved ( n 1 and n 2 – main quantum numbers).
Photon energy is expressed by the formula
= hc/
. Therefore, multiplying both sides of equality (1) by hc, we obtain an expression for the photon energy 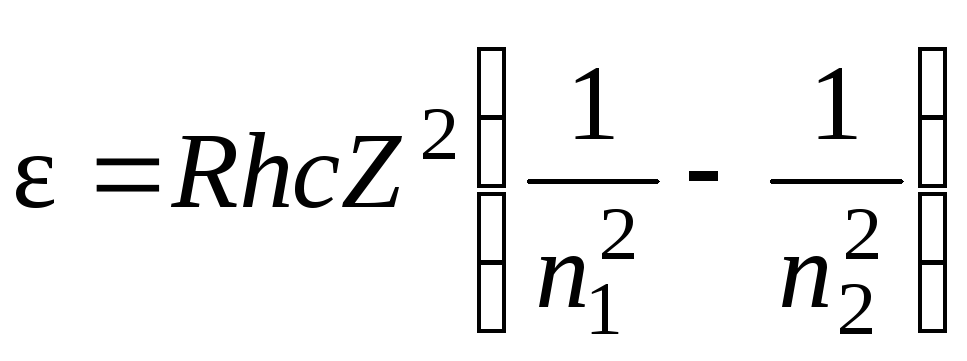 . Since the value Rhc- is the ionization energy E 1 hydrogen atom, then
. Since the value Rhc- is the ionization energy E 1 hydrogen atom, then 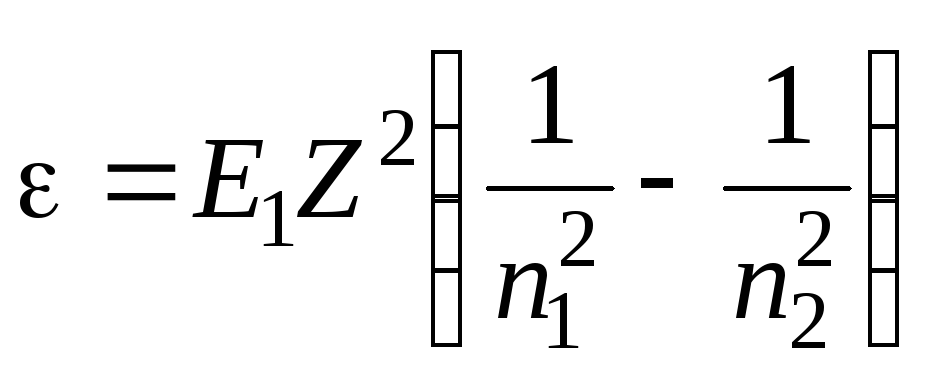 .
.
We will perform the calculations in non-system units: E 1 = 13.6 eV; Z= 1 (charge of the nucleus of a hydrogen atom in relative units, where the absolute value of the electron charge is taken as a unit of charge); n 1 =2; n 2 = 4;
№ 2. An electron, whose initial speed can be neglected, has passed through an accelerating potential difference U. Find the de Broglie wavelength for two cases: 1) U 1 = 51 V; U 2 = 510 kV.
Solution.
The de Broglie wavelength for a particle depends on its momentum R and is determined by the formula:
= h/r, (1)
Where h– Planck’s constant.
The momentum of a particle can be determined if its kinetic energy is known T. The relationship between momentum and kinetic energy is different for the nonrelativistic case (when the kinetic energy of the particle is much less than its rest energy) and for the relativistic case (when the kinetic energy is comparable to the rest energy of the particle).
We list the main characteristics of the cores, which will be discussed further:
- Binding energy and nuclear mass.
- Kernel sizes.
- Nuclear spin and angular momentum of the nucleons that make up the nucleus.
- Parity of the nucleus and particles.
- Isospin of the nucleus and nucleons.
- Spectra of nuclei. Characteristics of the ground and excited states.
- Electromagnetic properties of the nucleus and nucleons.
1. Binding energies and nuclear masses
The mass of stable nuclei is less than the sum of the masses of the nucleons included in the nucleus; the difference between these values determines the binding energy of the nucleus:
| (1.7) |
The coefficients in (1.7) are selected from the conditions for the best agreement between the model distribution curve and the experimental data. Since such a procedure can be carried out in different ways, there are several sets of Weizsäcker formula coefficients. The following are often used in (1.7):
a 1 = 15.6 MeV, a 2 = 17.2 MeV, a 3 = 0.72 MeV, a 4 = 23.6 MeV,

It is easy to estimate the value of the charge number Z at which nuclei become unstable with respect to spontaneous decay.
Spontaneous nuclear decay occurs when the Coulomb repulsion of nuclear protons begins to dominate over the nuclear forces pulling the nucleus together. An assessment of the nuclear parameters at which such a situation occurs can be made by considering changes in the surface and Coulomb energies during nuclear deformation. If the deformation leads to a more favorable energetic state, the nucleus will spontaneously deform until it divides into two fragments. Quantitatively, such an assessment can be carried out as follows.
During deformation, the core, without changing its volume, turns into an ellipsoid with axes (see Fig. 1.2 )
.
The binding energy E of the nucleus (A,Z) is the difference expressed in energy units between the mass M(A,Z) of the nucleus and the sum of the masses (A-Z) of neutrons and Zprotons:
E St (A , Z) = [(A - Z)M n + ZM p) - M ( A,Z ) ]c 2 .
The binding energy of the nucleus Eb determines the minimum energy that must be expended to divide the nucleus into individual nucleons.
Based on the analogy between a charged liquid drop of radius R = R 0 A 1/3 (where R 0 = 1.3 fm) and the atomic nucleus, K. Weizsäcker in 1935 wrote a semi-empirical formula for the binding energy of a nucleus:
The values of the coefficients a 1 - a 5 were selected so as to best reproduce the experimental values of the masses of stable nuclei:
a 1 = 15.6 MeV, a 2 = 17.2 MeV, a 3 = 0.72 MeV, a 4 = 23.6 MeV,

Binding energy E St (A,Z )
increases with increasing mass number A, reaching the value
~ 2 GeV in the region of mass numbers A~ 270. Therefore, it is much more convenient to use specific energy communications
ε = Eb /A - binding energy per nucleon (Fig. 2). The specific binding energy for most nuclei is ~8 MeV. The proportionality of the total binding energy to the number of nucleons in the nucleus is explained by the fact that nuclear forces are short-range and have the property of saturation.
Within the framework of the droplet model of the nucleus, it was possible to explain many properties of atomic nuclei and obtain a semi-empirical formula for the binding energy of atomic nuclei, which made it possible to understand some patterns of α- and β-decays, nuclear fission processes and to estimate the masses and binding energies of nuclei.
Radioactivity is the ability of an atomic nucleus to spontaneously decay by emitting particles.
Radioactive decay of a nucleus is possible when it is energetically favorable, i.e. accompanied by the release of energy. The condition for this is that the mass M of the original nucleus exceeds the sum of the masses m i of the decay products, which corresponds to the inequality M > ∑m i . This condition is necessary, but not always sufficient. Decay may be prohibited by other conservation laws - conservation of angular momentum, electric charge, baryon charge, etc.
Radioactive decay is characterized by the lifetime of the radioactive isotope, the type of particles emitted, and their energies.
The main types of radioactive decay are:
- α-decay – emission of α-particles by atomic nuclei;
- β-decay – emission of an electron and antineutrino, positron and neutrino by atomic nuclei, absorption of an atomic electron by the nucleus with neutrino emission;
- γ-decay – emission of γ-quanta by atomic nuclei;
- spontaneous fission - the disintegration of an atomic nucleus into two fragments of comparable mass.
Rarer types of radioactive decay include processes where nuclei emit two electrons, one or two protons, and clusters– light nuclei from 12 C to 32 S. In all types of radioactivity (except for γ-decay), the composition of the nucleus changes - the number of protons Z, the mass number A, or both at the same time.
The characteristics of radioactive decay are significantly influenced by the type of interaction that causes nuclear decay. Thus, α-decay is caused by strong interaction, β-decay by weak interaction, and γ-decay by electromagnetic interaction.
Radioactive decay is a statistical process. Each radioactive nucleus can decay at any moment, and the patterns of decay of an atomic nucleus are observed only on average; in the case of decay, quite large quantity cores.
To characterize the rate (probability) of radioactive decay, three interrelated quantities are used - the decay constant λ, the average lifetime t and the half-life T 1/2.
Decay constantλ is the probability of nuclear decay per unit time. If there are N radioactive nuclei in a sample at time t, then the number of nuclei dN that decayed during time dt is proportional to N, λ and the time interval dt:
The law of radioactive decay has the form:
N(t) = N 0 e -λt ,
where N 0 is the number of radioactive nuclei at the initial time t = 0.
Average life timeτ:
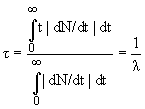 .
.
Half life T 1/2 – time during which the initial number of radioactive nuclei is halved:
T 1/2 = ln2/λ=0.693/λ = τln2.
Dimensions and shape of the kernel. For the first time, the size of the nucleus was correctly estimated by Rutherford, using the scattering of alpha particles for this purpose. His first experiments showed that the dimensions of the charged part of the nucleus are about 10–14 m. Later and more accurate experiments made it possible to establish that the radius of the nucleus is approximately proportional to A 1/3 and, therefore, the density of nuclear matter is almost constant. (It is colossal: 100,000 t/mm 3.)
With the discovery of the neutron, it became clear that it represents an ideal means of studying the nucleus, since neutral particles, passing at a considerable distance from the nucleus, are not deflected by the nuclear charge. In other words, a neutron collides with a nucleus if the distance between their centers is less than the sum of their radii, and otherwise does not deflect. Experiments on scattering a neutron beam have shown that the radius of the nucleus (assuming a spherical shape) is equal to:
R = r 0 A 1/3 ,
r 0 » 1.4×10 –15 m.
Thus, the radius of the uranium-238 nucleus is 8.5 × 10 –15 m. The resulting value corresponds to the radius of action of nuclear forces; it characterizes the distance from the center of the nucleus at which the external neutral nucleon begins to “feel” its influence for the first time. This value of the nuclear radius is comparable to the distance from the center of the nuclei at which alpha particles and protons are scattered.
The scattering of alpha particles, protons and neutrons by nuclei is due to the action of nuclear forces; Consequently, such measurements of nuclear radii provide an estimate of the radius of action of nuclear forces. The interaction of electrons with nuclei is almost completely determined by electrical forces. Therefore, electron scattering can be used to study the shape of the charge distribution in the nucleus. Experiments with very high energy electrons conducted by R. Hofstadter at Stanford University provided detailed information about the distribution of positive charge along the radius of the nucleus. In Fig. Figure 6 shows the angular distribution of electrons with an energy of 154 MeV scattered by gold nuclei. The upper curve characterizes the angular distribution calculated under the assumption that the positive charge is concentrated at a point; It is obvious that the experimental data do not correspond to this assumption. Much better agreement is achieved under the assumption of a uniform distribution of protons throughout the volume of the nucleus (lower curve). However, the “charge radius” turns out to be approximately 20% smaller than the “nuclear force” radius obtained from neutron scattering data. This may mean that the distribution of protons in the nucleus is different from the distribution of neutrons.
1. General properties of atomic nuclei. Rutherford's experiments established the existence of atomic nuclei. The atomic nucleus of each element has certain properties that determine: electric charge, mass, electric and magnetic moments, spin, etc. Core charge. Electric charge atomic nucleus is positive. Its value is determined by the product Ze, where Z is the atomic number of the element equal to the serial number in the periodic system of D.I. Mendeleev, e is the elementary charge equal to 1.6022 1019 C. The electric charge determines the number of protons in the nucleus and the number of electrons in a neutral atom, the nature of the intra-atomic electric field, on which the physical and chemical properties of atoms depend. The mass of the nucleus is its second important characteristic. In practice, the mass of the atomic nucleus coincides with the mass of the atom, since the mass of the electrons that make up the atom is very insignificant. The mass of atoms can be determined by the deviation of their ions in electrical and magnetic fields. Atoms whose nuclei have the same charges but different masses are called isotopes.
Sizes and shape of kernels. Certain information about the size and shape of a nucleus can be obtained by studying its electric field, which is studied by the method of scattering charged particles on nuclei. The study of the electric field of the nucleus allowed us to draw a conclusion about its shape. So, in the case of a spherically symmetric nucleus, its field should also be spherically symmetric, that is, the same as the field point charge. The studies have shown that not all nuclei are spherically symmetrical, but all nuclei, without exception, are characterized by axial symmetry.
The spin of a nucleus, together with its charge and mass, is its the most important characteristic. The spin of a nucleus is called its total mechanical moment, which is the sum of the intrinsic angular momentum of its constituent particles and their orbital mechanical moments caused by intranuclear motions. The spin of the nucleus depends on its state. Therefore, the spin of the nucleus in the ground state is usually assumed. The nuclear spin is determined by the number of hyperfine structure lines during spectroscopic studies. In addition to spin, nuclei have characteristic magnetic moments. The magnetic moments of nuclei are expressed in nuclear magnetons, which are introduced similarly to the Bohr magneton. There is an unambiguous connection between the spin and the statistics of the nucleus. Nuclei with integer spin are subject to Fermi-Dirac statistics, and with integer spin - to Bose-Einstein statistics.
2. Nuclear binding energy. Specific binding energy. The energy that must be expended in order to overcome nuclear forces and split a nucleus into individual nucleons is called binding energy atomic nucleus. As follows from the law of conservation of energy, if a nucleus is formed from individual nucleons, then the binding energy of the nucleus at the moment of its formation is released in the form of radiation. From the law of the relationship between mass and energy it follows that E St. =Dm·c 2 , Where Dm-nucleus mass defect.
Let's calculate the total rest mass of nucleons entering the nucleus of any element: (Z·m p +(A-Z)·m n). Let's compare the resulting number with the mass of the nucleus M i. It turned out that for all elements of the periodic table the mass of the nucleus is less than the total mass of the particles that make up the nucleus. The difference between these values is called the mass defect:
Dm=Z m p +(A-Z) m n -M I
So, the formula by which you can calculate the binding energy is:
E St. =(Z m p +(A-Z) m n -M I )·c 2
The binding energy per nucleon is called specific energy communications: dE=DE/A
In Fig. Figure 20 shows a graph of specific binding energy versus mass number. Analyzing this graph, we can draw the following conclusions:
1. Specific binding energy is not a constant value for different nuclei, i.e. The bond strength of nucleons in different nuclei is different. The nucleons are most tightly bound in nuclei with mass numbers in the range of approximately 40 to 100. For this group of nuclei, the specific binding energy is approximately 8.7 MeV/nucleon.
2. The specific binding energy of nuclei with mass number A > 100 decreases and for uranium is 7.6 MeV.
3. In light nuclei, the specific binding energy decreases with decreasing number of nucleons in the nucleus. A characteristic feature of the specific binding energy curve in this group of nuclei is the presence of sharp maxima and minima. The maximum value of the specific binding energy falls on the nuclei and the minimum value falls on the nuclei

3. Weizsäcker formula for binding energy.
Bond energy:
E St =c 2. (1)
It is more convenient to use the following notation (accurate to the electron binding energy):
Let us consider the ratio of the binding energy of the nucleus to the mass number
By definition, ε is average binding energy, per one nucleon ( specific binding energy nucleon in the nucleus). Thus, it characterizes the intensity of nuclear forces. As can be seen from Fig. 1, at small values of mass numbers ε increases sharply and reaches a maximum at A ≈ 5060 (about 8.38.8 MeV). Nuclides with such mass numbers are the most stable. With further growth of A, the average binding energy decreases, however, over a wide range of mass numbers, the value of the specific binding energy is almost constant (=8 MeV). From the above it follows that we can write (3)
It is not difficult to understand that if each nucleon of the nucleus interacted with (A– 1) other nucleons, then total energy of this interaction would be proportional to the product A(A – 1) ≈ A. The difference between this relationship and (3) indicates the property of saturation of nuclear forces: each nucleon in the nucleus interacts not with all the others, but only with a limited number of neighboring nucleons. Nuclear forces are attractive forces, and, as evidenced by the existence of stable nuclei, under some conditions they are greater than the Coulomb repulsion forces (the Coulomb repulsion energy of two neighboring protons in a nucleus is an order of magnitude less than the attractive energy).
Dependence of the average binding energy ε per nucleon on the mass number
 Fig.1
Fig.1
The release of energy in nuclear fusion or fission reactions is due to an increase in ε during the fusion of the lightest nuclei into heavier ones or during the fission of heavy nuclei. Local maxima of the ε(A) curve are associated with the formation of stable nuclear shells.
The form of the dependence of the binding energy on the mass number led to the idea of an analogy between a nucleus and a drop of liquid, which led to the creation of a droplet model of the nucleus and the obtaining of a semi-empirical Weizsäcker formulas for the nuclear binding energy.
 ,
,
where a 1 = 15.75 MeV; a 2 = 17.8 MeV; a 3 = 0.71 MeV; a 4 = 23.7 MeV; │δ│ = 34 A -3/4. The first term determines the proportionality of the binding energy of the nucleus and the mass of the nucleus, the equivalence of the nucleons of the nucleus and the interaction of each of them only with nearby neighbors. The second term takes into account the fact that nucleons on the surface of the nucleus interact with a smaller number of other nucleons and are thus bound to them less strongly (evaporation of the molecules of a liquid drop occurs from its surface). This leads to a decrease in the binding energy of the nucleus. The total number of “surface” nucleons is proportional to R 2 ~ A 2/3. The third term takes into account the presence of Coulomb repulsion forces between protons (ΔE coul ~ Z(Z – 1)/R ≈ Z 2 /R ~ Z 2 /A 1/3). The fourth term takes into account the presence of proton-neutron asymmetry (presence of spin). The fifth term takes into account the influence of the parity of Z and A – Z on the stability of nuclei: for even-even nuclei (even A and even Z) is substituted into the formula +│δ│; for odd-odd nuclei (even A and odd Z) is substituted into the formula -│δ│; for odd-even and even-odd kernels (all other options), 0 is substituted into the Weizsäcker formula.
4. Weizsäcker formula for core mass.
If you buy 5 apples, 200 g each, put them in a bag, and then, after weighing it, you see that you have less than 1 kg of apples, you will, of course, be surprised, but you will not suspect “nature” of deception. You will decide that the seller deceived you, that the apples that you were promised weigh 200 g are actually lighter. But if a physicist, having measured the mass of the nucleus, finds that it is less than the sum of the masses of the nucleons that make up the nucleus, he, too, will not suspect “nature” of deception, he will not even be surprised. He knows that this is due to the interaction between particles.
Rest energy of a composite system equal to the sum of the rest energies of its constituent particles, their kinetic energy and interaction energy. The kinetic energy of the particles that make up the system is less than the energy of their interaction (the latter is negative), otherwise the particles would scatter far from each other. Therefore, the rest energy of a composite system is less than the sum of the rest energies of its constituent particles. So, in accordance with Einstein’s formula
E=mc 2
mass of the composite system is also less than the sum of the masses of its constituent particles. Where does the energy go during formation? connected system? The answer to the form is very simple - it radiates. But if we wanted to describe the radiation process in detail, we would have to turn to complex field theories. Let's return to our apples. Why are we sure that the mass of a bag of apples should be equal to the sum of the masses of all the apples and, of course, the bag? Simply because the apples did not form a bound state.
Core mass m less than the masses of the nucleons that make up the nucleus by the amount Δm, called mass defect:
Δm = Zm p +Nm n — m,
Where m p And m n— masses of proton and neutron.
Energy of communication E NE kernels is the difference between the rest energies of the nucleus and the nucleons that make up the nucleus:
ENE =Zm p c 2 + Nm n c 2 — mc 2 = Δmc 2 .
This is exactly the energy that needs to be expended in order to split the nucleus into separate nucleo-clones. For most but the lightest kernels, binding energy is approximately proportional to the number of nucleons in the nucleus, and the specific binding energy
ε SV =E NE /A
is almost constant and amounts to ~6-8 MeV/nucleon. This property is called saturation of nuclear forces. It indicates that a nucleon in a nucleus interacts effectively only with a small number of other nucleons. If each nucleon interacted with all nucleons, then the specific binding energy would be proportional to the number of nucleons in the nucleus A.
Relative nuclear mass defect:
Δm/m =ENE / m p c 2 .
For most nuclei it is ~0.65-0.85%; for light nuclei it is less, for example, for the deuteron it is 0.1%. Material from the site
On this page there is material on the following topics:
