Liquids tend to evaporate. If we dropped a drop of water, ether and mercury onto the table (just don’t do this at home!), we could observe how the drops gradually disappear - evaporate. Some liquids evaporate faster, others slower. The process of evaporation of liquid is also called vaporization. And the reverse process of turning steam into liquid is condensation.
These two processes illustrate phase transition– the process of transition of substances from one state of aggregation to another:
- evaporation (transition from liquid to gaseous state);
- condensation (transition from a gaseous state to a liquid);
- desublimation (transition from a gaseous state to a solid state, bypassing the liquid phase);
- sublimation, also known as sublimation (transition from solid to gaseous state, bypassing liquid).
Now, by the way, is the right season to observe the process of desublimation in nature: frost and hoarfrost on trees and objects, frosty patterns on windows - its result.
How saturated and unsaturated steam is formed
But let's return to vaporization. We will continue to experiment and pour liquid - water, for example, into an open vessel, and connect a pressure gauge to it. Invisible to the eye, evaporation occurs in the vessel. All molecules of a liquid are in continuous movement. Some move so fast that their kinetic energy is stronger than what binds the liquid molecules together.
Having left the liquid, these molecules continue to move chaotically in space, the vast majority of them are scattered in it - this is how unsaturated steam. Only a small part of them returns back to the liquid.
If we close the vessel, the number of vapor molecules will gradually increase. And more and more of them will return to the liquid. This will increase the steam pressure. This will be recorded by a pressure gauge connected to the vessel.
After some time, the number of molecules flying out of the liquid and returning to it will be equal. The steam pressure will stop changing. As a result steam saturation thermodynamic equilibrium of the liquid-vapor system will be established. That is, evaporation and condensation will be equal.
Properties of saturated steam
To illustrate them clearly, we use another experiment. Use all the power of your imagination to imagine it. So, let's take a mercury manometer, consisting of two elbows - communicating tubes. Both are filled with mercury, one end is open, the other is sealed, and above the mercury there is still a certain amount of ether and its saturated vapor. If you lower and raise the unsealed knee, the mercury level in the sealed one will also fall and rise.
In this case, the amount (volume) of saturated ether vapor will also change. The difference in the levels of mercury columns in both legs of the manometer shows the saturated vapor pressure of the ether. It will remain unchanged all the time.
This implies the property of saturated steam - its pressure does not depend on the volume it occupies. Pressure saturated vapors different liquids (water and ether, for example) are different at the same temperature.
However, the temperature of the saturated steam matters. The higher the temperature, the higher the pressure. The pressure of saturated steam increases with increasing temperature faster than it does with unsaturated steam. The temperature and pressure of unsaturated steam are related linearly.
Another interesting experiment can be done. Take an empty flask without liquid vapor, close it and connect the pressure gauge. Gradually, drop by drop, add liquid into the flask. As the liquid enters and evaporates, the saturated vapor pressure is established, the highest for a given liquid at a given temperature.
More about temperature and saturated steam
The temperature of the steam also affects the rate of condensation. Just as the temperature of a liquid determines the rate of evaporation - the number of molecules that fly out from the surface of the liquid per unit time, in other words.
For saturated steam, its temperature is equal to the temperature of the liquid. The higher the temperature of the saturated vapor, the higher its pressure and density, the lower the density of the liquid. When the critical temperature for a substance is reached, the density of the liquid and vapor is the same. If the vapor temperature is higher than the critical temperature for the substance, the physical differences between the liquid and saturated vapor are erased.
Determination of saturated vapor pressure in a mixture with other gases
We talked about the saturated vapor pressure being constant at a constant temperature. We determined the pressure under “ideal” conditions: when a vessel or flask contains liquid and vapor of only one substance. Let us also consider an experiment in which molecules of a substance are scattered in space in a mixture with other gases.
To do this, take two open glass cylinders and place closed vessels with ether in both. As usual, let's connect the pressure gauges. We open one vessel with ether, after which the pressure gauge records the increase in pressure. The difference between this pressure and the pressure in a cylinder with a closed vessel of ether allows us to find out the pressure of the saturated vapor of ether.
About pressure and boiling
Evaporation is possible not only from the surface of the liquid, but also in its volume - then it is called boiling. As the temperature of the liquid increases, vapor bubbles form. When the saturated vapor pressure is greater than or equal to the gas pressure in the bubbles, the liquid evaporates into the bubbles. And they expand and rise to the surface.
Liquids boil at different temperatures. Under normal conditions, water boils at 100 0 C. But with a change in atmospheric pressure, the boiling point also changes. So, in the mountains, where the air is very rarefied and the atmospheric pressure is lower, as you rise into the mountains the boiling point of water decreases.
By the way, boiling in a hermetically sealed vessel is impossible at all.
![]()
Another example of the relationship between vapor pressure and evaporation is demonstrated by such a characteristic of the content of water vapor in the air as relative air humidity. It is the ratio of the partial pressure of water vapor to the saturated vapor pressure and is determined by the formula: φ = r/r o * 100%.
As the air temperature decreases, the concentration of water vapor in it increases, i.e. they become more saturated. This temperature is called the dew point.
Let's sum it up
Using simple examples, we analyzed the essence of the evaporation process and the unsaturated and saturated steam formed as a result. You can observe all these phenomena around you every day: for example, see puddles drying up on the streets after rain or a mirror fogged up from steam in the bathroom. In the bathroom, you can even observe how steam formation first occurs, and then the moisture accumulated on the mirror condenses back into water.
You can also use this knowledge to make your life more comfortable. For example, in winter the air in many apartments is very dry, and this has a bad effect on well-being. You can use a modern humidifier device to make it more humid. Or, in the old fashioned way, place a container of water in the room: gradually evaporating, the water will saturate the air with its vapors.
www.site, when copying material in full or in part, a link to the source is required.
The processes of evaporation and condensation occur continuously and parallel to each other.
In an open vessel, the amount of liquid decreases over time, because evaporation predominates over condensation.
The vapor that exists above the surface of a liquid when evaporation prevails over condensation or vapor in the absence of liquid is called unsaturated.
In a hermetically sealed vessel, the liquid level does not change over time, because evaporation and condensation compensate each other: as many molecules fly out of the liquid, the same number of them return to it at the same time, and a dynamic (mobile) equilibrium occurs between the vapor and its liquid.
Vapor that is in dynamic equilibrium with its liquid is called saturated.
At a given temperature, the saturated vapor of any liquid has the highest density ( ) and creates maximum pressure ( ) that the vapor of this liquid can have at this temperature.
The pressure and density of saturated vapor at the same temperature depends on the type of substance: greater pressure creates saturated vapor of the liquid that evaporates faster. For example, and
Properties of unsaturated vapors: Unsaturated vapors obey the gas laws of Boyle - Mariotte, Gay-Lussac, Charles, and the equation of state of an ideal gas can be applied to them.
Properties of saturated vapors:1. At a constant volume, with increasing temperature, the pressure of saturated vapor increases, but not directly proportionally (Charles’ law is not satisfied), the pressure increases faster than that of an ideal gas. , with increasing temperature ( ) , the mass of steam increases, and therefore the concentration of steam molecules increases () and the pressure of saturated steam will melt for two reasons (
3 1 – unsaturated steam (ideal gas);
2 2 - saturated steam; 3 – unsaturated steam,
1 obtained from saturated steam in the same
Volume when heated.
2. The pressure of saturated steam at a constant temperature does not depend on the volume it occupies.
As the volume increases, the mass of the vapor increases, and the mass of the liquid decreases (part of the liquid turns into steam); when the volume decreases, the vapor becomes less and the liquid becomes larger (part of the vapor turns into liquid), while the density and concentration of the molecules of saturated vapor remain constant, therefore, the pressure remains constant ().
liquid
(sat. steam + liquid)
Unsaturated steam
Saturated vapors do not obey the gas laws of Boyle - Mariotte, Gay-Lussac, Charles, because the mass of steam in processes does not remain constant, but everything gas laws obtained for constant mass. The ideal gas equation of state can be applied to saturated steam.
So, saturated steam can be converted to unsaturated steam either by heating it at a constant volume or by increasing its volume at a constant temperature. Unsaturated steam can be converted to saturated steam either by cooling it at a constant volume or by compressing it at a constant temperature.
Critical condition
The presence of a free surface of a liquid makes it possible to indicate where the liquid phase of a substance is located and where the gaseous phase is. The sharp difference between a liquid and its vapor is explained by the fact that the density of the liquid is many times greater than that of the vapor. If you heat a liquid in a hermetically sealed vessel, then due to expansion its density will decrease, and the density of the vapor above it will increase. This means that the difference between the liquid and its saturated vapor is smoothed out and at a sufficiently high temperature disappears completely. The temperature at which differences in physical properties ah between the liquid and its saturated vapor, and their densities become the same, is calledcritical temperature.
Critical point
For the formation of liquid from gas, the average potential energy the attraction of molecules must exceed their average kinetic energy.
Critical temperature – the maximum temperature at which steam turns into liquid. The critical temperature depends on the potential energy of interaction between molecules and is therefore different for different gases. Due to the strong interaction of water molecules, water vapor can be converted into water even at temperatures of . At the same time, nitrogen liquefaction occurs only at a temperature lower than = -147˚, because nitrogen molecules interact weakly with each other.
Another macroscopic parameter that affects the vapor-liquid transition is pressure. With increasing external pressure during gas compression, the average distance between particles decreases, the force of attraction between them increases and, accordingly, the average potential energy of their interaction increases.
Pressuresaturated steam at its critical temperature is called critical. This is the highest possible saturated vapor pressure of a given substance.
State of matter with critical parameters is called critical(critical point) . Each substance has its own critical temperature and pressure.
In a critical state, the specific heat of vaporization and the coefficient of surface tension of the liquid vanish. At temperatures above critical, even at very high pressures, the transformation of gas into liquid is impossible, i.e. Liquid cannot exist above the critical temperature. At supercritical temperatures, only the vapor state of the substance is possible.
Liquefaction of gases is possible only at temperatures below the critical temperature. To liquefy, gases are cooled to a critical temperature, such as through adiabatic expansion, and then compressed isothermally.
Boiling
Externally the phenomenon looks like this: Rapidly growing bubbles rise from the entire volume of the liquid to the surface, burst on the surface, and steam is released into the environment.
MKT explains boiling as follows: There are always air bubbles in a liquid; evaporation occurs in them. The closed volume of the bubbles turns out to be filled not only with air, but also with saturated steam. When the liquid is heated, the saturated vapor pressure in them increases faster than the air pressure. When, in a sufficiently heated liquid, the saturated vapor pressure in the bubbles becomes greater than the external pressure, they increase in volume, and a buoyant force that exceeds their gravity lifts the bubbles to the surface. The floating bubbles begin to burst when, at a certain temperature, the pressure of the saturated vapor in them exceeds the pressure above the liquid. The temperature of a liquid at which the pressure of its saturated vapor in the bubbles is equal to or exceeds the external pressure on the liquid is called boiling point.
The boiling point of different liquids is different, because the saturated vapor pressure in their bubbles is compared with the same external pressure at different temperatures. For example, the pressure of saturated vapor in bubbles is equal to normal atmospheric pressure for water at 100˚C, for mercury at 357˚C, for alcohol at 78˚C, for ether at 35˚C.
The boiling point remains constant during the boiling process, because all the heat that is supplied to the heated liquid is spent on vaporization.
The boiling point depends on the external pressure on the liquid: with increasing pressure, the temperature rises; As the pressure decreases, the temperature decreases. For example, at an altitude of 5 km above sea level, where the pressure is 2 times lower than atmospheric pressure, the boiling point of water is 83˚C, in the boilers of steam engines, where the steam pressure is 15 atm. (), water temperature is about 200˚С.
Air humidity
There is always water vapor in the air, so we can talk about air humidity, which is characterized by the following values:
1.Absolute humidity is the density of water vapor in the air (or the pressure that this vapor creates (.
Absolute humidity does not give an idea of the degree of saturation of air with water vapor. The same amount of water vapor at different temperatures creates a different feeling of humidity.
2.Relative humidity- is the ratio of the density (pressure) of water vapor contained in the air at a given temperature to the density (pressure) of saturated vapor at the same temperature : or
– absolute humidity at a given temperature; - density, saturated vapor pressure at the same temperature. The density and pressure of saturated water vapor at any temperature can be found in the table. The table shows that the higher the air temperature, the greater the density and pressure of water vapor in the air must be for it to be saturated.
Knowing the relative humidity, you can understand by what percentage the water vapor in the air at a given temperature is far from saturation. If the vapor in the air is saturated, then . If , then there is not enough steam in the air to reach a state of saturation.
The fact that steam in the air becomes saturated is judged by the appearance of moisture in the form of fog or dew. The temperature at which water vapor in the air becomes saturated is called dew point.
Vapor in the air can be made saturated by adding vapor through additional evaporation of the liquid without changing the air temperature, or if there is an amount of vapor in the air, lower its temperature.
Normal relative humidity, most favorable for humans, is 40 - 60%. Great importance has knowledge of humidity in meteorology for weather prediction. In weaving and confectionery production, a certain humidity is required for the normal course of the process. Storing works of art and books requires maintaining air humidity at the required level.
Instruments for determining humidity:
1. Condensation hygrometer (allows you to determine the dew point).
2. Hair hygrometer (the principle of operation is based on the dependence of the length of fat-free hair on humidity) measures relative humidity as a percentage.
3. The psychrometer consists of two thermometers, dry and humidified. The reservoir of the moistened thermometer is wrapped in a cloth dipped in water. Due to evaporation from the fabric, the temperature of the moistened one is lower than that of the dry one. The difference in thermometer readings depends on the humidity of the surrounding air: the drier the air, the more intense the evaporation from the fabric, the greater the difference in thermometer readings and vice versa. If the air humidity is 100%, then the thermometer readings are the same, i.e. the difference in readings is 0. To determine humidity using a psychrometer, a psychrometric table is used.
Melting and crystallization
When melting solid body, the distance between the particles forming the crystal lattice increases, and the lattice itself is destroyed. The melting process requires energy. When a solid body is heated, the kinetic energy of vibrating molecules increases and, accordingly, the amplitude of their vibrations. At a certain temperature, called melting point, the order in the arrangement of particles in the crystals is disrupted, the crystals lose their shape. A substance melts, changing from a solid state to a liquid state.
Upon crystallization Molecules come together to form a crystal lattice. Crystallization can only occur when the liquid releases energy. As the molten substance cools, the average kinetic energy and speed of the molecules decrease. Attractive forces can hold particles near their equilibrium positions. At a certain temperature, called temperature of solidification (crystallization), all molecules find themselves in a position of stable equilibrium, their arrangement becomes ordered - a crystal is formed.
Melting of a solid occurs at the same temperature at which the substance solidifies
Each substance has its own melting point. For example, the melting point of helium is -269.6˚C, mercury is -38.9˚C, and copper is 1083˚C.
During the melting process the temperature remains constant. The amount of heat supplied from outside is used to destroy the crystal lattice.
During the curing process, although heat is removed, the temperature does not change. The energy released during crystallization is spent on maintaining a constant temperature.
Until the whole substance melts or the whole substance hardens, i.e. As long as the solid and liquid phases of a substance exist together, the temperature does not change.
TV+liquid liquid+tv
, where is the amount of heat, is the amount of heat required to melt the substance released during crystallization of the substance by mass
- specific heat of fusion– the amount of heat required to melt a substance weighing 1 kg at its melting point.
What amount of heat is expended during the melting of a certain mass of a substance, the same amount of heat is released during the crystallization of this mass.
Also called specific heat of crystallization.
At melting point internal energy a substance in a liquid state has more internal energy than the same mass of a substance in a solid state.
U large number When substances melt, their volume increases and their density decreases. When hardening, on the contrary, the volume decreases and the density increases. For example, crystals of solid naphthalene sink in liquid naphthalene.
Some substances, for example, bismuth, ice, gallium, cast iron, etc., compress when melting, and expand when solidifying. These deviations from general rule explained by the structural features of crystal lattices. Therefore, water turns out to be denser than ice, ice floats in water. The expansion of water when it freezes leads to the destruction of rocks.
The change in volume of metals during melting and solidification is of significant importance in foundry.
Experience shows that change in external pressure by solid is reflected in the melting point of this substance. For those substances that expand during melting, an increase in external pressure leads to an increase in the melting temperature, because complicates the melting process. If substances are compressed during melting, then for them an increase in external pressure leads to a decrease in the melting temperature, because helps the melting process. Only a very large increase in pressure noticeably changes the melting point. For example, to lower the melting temperature of ice by 1˚C, the pressure needs to be increased by 130 atm. The melting point of a substance at normal atmospheric pressure called melting point of a substance.
If an open glass of water is left on for a long time, then eventually the water will completely evaporate. More precisely, it will evaporate. What is evaporation and why does it happen?
2.7.1 Evaporation and condensation
At a given temperature, liquid molecules have different speeds. The velocities of most molecules are close to a certain average value (characteristic of this temperature). But there are molecules whose speeds differ significantly from the average, both smaller and larger.
In Fig. Figure 2.16 shows an approximate graph of the velocity distribution of liquid molecules. The blue background shows the majority of molecules whose velocities are grouped around the average value. The red “tail” of the graph is a small number of “fast” molecules, the speeds of which significantly exceed the average speed of the bulk of liquid molecules.
Number of molecules
Fast molecules
Speed of molecules
Rice. 2.16. Distribution of molecules by speed
When such a very fast molecule finds itself on the free surface of the liquid (i.e., at the interface between liquid and air), the kinetic energy of this molecule may be enough to overcome the attractive forces of other molecules and fly out of the liquid. This process and there is evaporation, and the molecules leaving the liquid form vapor.
So, evaporation is the process of converting a liquid into vapor, occurring on the free surface of the liquid7.
It may happen that after some time the vapor molecule returns back to the liquid.
The process of vapor molecules changing into liquid is called condensation. Vapor condensation is the reverse process of liquid evaporation.
2.7.2 Dynamic balance
What happens if a vessel with liquid is hermetically sealed? The vapor density above the liquid surface will begin to increase; vapor particles will increasingly interfere with other liquid molecules flying out, and the evaporation rate will begin to decrease. At the same time it will start
7 Under special conditions, the transformation of liquid into vapor can occur throughout the entire volume of the liquid. This process is well known to you - boiling.
p n = n RT:
the condensation rate will increase, since with increasing vapor concentration the number of molecules returning to the liquid will increase.
Finally, at some point the rate of condensation will be equal to the rate of evaporation. A dynamic equilibrium will occur between liquid and vapor: per unit time, the same number of molecules will fly out of the liquid as return to it from the vapor. From this moment on, the amount of liquid will cease to decrease, and the amount of vapor will cease to increase; steam will reach ¾saturation¿.
Saturated steam It is a vapor that is in a state of dynamic equilibrium with its liquid. Vapor that has not reached a state of dynamic equilibrium with the liquid is called unsaturated.
The pressure and density of saturated steam are designated pн in. Obviously, pn in is the maximum pressure and density that steam can have at a given temperature. In other words, the pressure and density of saturated steam always exceeds the pressure and density of unsaturated steam.
2.7.3 Properties of saturated steam
It turns out that the state of saturated steam (and even more so of unsaturated steam) can be approximately described by the equation of state of an ideal gas (Mendeleev-Clapeyron equation). In particular, we have an approximate relationship between saturated vapor pressure and its density:
This is a very surprising fact, confirmed by experiment. Indeed, in its properties, saturated steam differs significantly from an ideal gas. Let us list the most important of these differences.
1. At a constant temperature, the density of saturated vapor does not depend on its volume.
If, for example, saturated steam is compressed isothermally, then its density will increase at the first moment, the condensation rate will exceed the evaporation rate, and part of the vapor will condense into liquid until dynamic equilibrium occurs again, in which the vapor density will return to its previous value.
Similarly, during isothermal expansion of saturated steam, its density will decrease at the first moment (the steam will become unsaturated), the rate of evaporation will exceed the rate of condensation, and the liquid will further evaporate until dynamic equilibrium is established again, i.e., until the steam again becomes saturated with the same density.
2. The pressure of saturated steam does not depend on its volume.
This follows from the fact that the density of saturated vapor does not depend on volume, and pressure is uniquely related to density by equation (2.6).
As we see, Boyle-Mariotte's law, which is valid for ideal gases, does not hold true for saturated steam. This is not surprising, since it was obtained from the Mendeleev-Clapeyron equation under the assumption that the mass of the gas remains constant.
3. At a constant volume, the density of saturated vapor increases with increasing temperature and decreases with decreasing temperature.
Indeed, as the temperature increases, the rate of liquid evaporation increases. The dynamic equilibrium is disrupted at the first moment, and additional
evaporation of some liquid. The pair will be added until dynamic equilibrium is restored again.
In the same way, as the temperature decreases, the rate of liquid evaporation becomes slower, and part of the vapor condenses until dynamic equilibrium is restored, but with less vapor.
Thus, when saturated steam is heated or cooled isochorically, its mass changes, so Charles’s law does not work in this case. The dependence of saturated vapor pressure on temperature will no longer be a linear function.
4. Saturated vapor pressure increases with temperature faster than according to a linear law.
In fact, with increasing temperature, the density of saturated vapor increases, and according to equation (2.6), the pressure is proportional to the product of density and temperature.
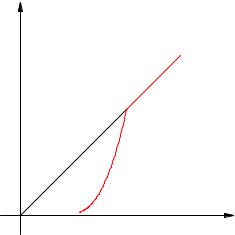
The dependence of saturated vapor pressure on temperature is exponential (Fig. 2.17). It is represented by section 1–2 of the graph. This dependence cannot be derived from the ideal gas laws.
isochore pair
Rice. 2.17. Dependence of steam pressure on temperature
At point 2 all liquid evaporates; with a further increase in temperature, the steam becomes unsaturated, and its pressure increases linearly according to Charles’s law (section 2–3).
Let us recall that the linear increase in pressure of an ideal gas is caused by an increase in the intensity of impacts of molecules on the walls of the vessel. When saturated steam is heated, the molecules begin to beat not only harder, but also more often because the steam becomes larger. The simultaneous action of these two factors causes an exponential increase in saturated vapor pressure.
2.7.4 Air humidity
Absolute humidity is the partial pressure of water vapor in the air (i.e., the pressure that water vapor would exert on its own, in the absence of other gases). Sometimes absolute humidity is also called the density of water vapor in the air.
Relative air humidity "is the ratio of the partial pressure of water vapor in it to the pressure of saturated water vapor at the same temperature. As a rule, this is
the ratio is expressed as a percentage:
" = p 100%: pн
From the Mendeleev-Clapeyron equation (2.6) it follows that the ratio of vapor pressures is equal to the ratio of densities. Since equation (2.6) itself, recall, describes saturated steam only approximately, we have an approximate relation:
" = 100%:n
One of the devices that measures air humidity is a psychrometer. It includes two thermometers, the reservoir of one of which is wrapped in a wet cloth. The lower the humidity, the more intense the evaporation of water from the fabric, the more the reservoir of the wet thermometer cools, and the greater the difference between its readings and the readings of the dry thermometer. From this difference, air humidity is determined using a special psychrometric table.
Topic 2. PHASE TRANSITIONS.
Phase transition (phase transformation) is the transition of a substance from one phase to another when external conditions change (for example, temperature, pressure, magnetic and electric fields, etc.), accompanied by a change in the physical properties and parameters of the substance.
The value of temperature, pressure or any other physical quantity at which a phase transition occurs is called the transition point. There are two types of phase transition.
PHASE TRANSITIONS OF THE FIRST ORDER
During a first-order phase transition, such thermodynamic parameters change abruptly
characteristics of a substance, such as density, concentration of components, specific volume, amount of stored internal energy, i.e. a certain amount of heat is released or absorbed, called the heat of transition. What is meant here is an abrupt change in these quantities not in time, but with changes in temperature, pressure, etc. The most common examples of first-order phase transitions are:
- melting and crystallization
- evaporation and condensation
- sublimation and desublimation
PHASE TRANSITIONS OF THE SECOND ORDER
During a second-order phase transition, the density and internal energy do not change, so such a phase transition may not be noticeable to the naked eye. The jump is experienced by their derivatives in temperature and pressure: heat capacity, coefficient of thermal expansion, various susceptibilities, etc. That is. second-order phase transitions are accompanied by a change in the symmetry of the structure of a substance, and not by the release or absorption of energy (heat). The most common examples of second-order phase transitions are:
- passage of the system through a critical point
- paramagnetic-ferromagnetic transition
- transition of metals and alloys to a state of superconductivity
- transition of liquid helium to a superfluid state
- transition of amorphous materials to a glassy state
Modern physics also studies systems that have phase transitions of the third or higher order. Recently, the concept of quantum phase transition has become widespread, i.e. a phase transition controlled not by classical thermal fluctuations, but by quantum ones that exist even at absolute zero temperatures where the classical phase transition cannot occur due to Nernst's theorem.
Let us consider in more detail the phenomena of interest to us associated with first-order phase transitions.
EVAPORATION, CONDENSATION, BOILING.
SATURATED AND UNSATURATED PAIRS.
Under certain conditions, any substance can be in various states of aggregation - solid, liquid and gaseous. Transitions from one state of aggregation to a second are phase transitions of the first order.
Evaporation And condensation are phase transitions between the liquid and gaseous phases of a substance.
All real gases(oxygen, nitrogen, hydrogen, etc.) under certain conditions are capable of turning into liquid. However, such a transformation can only occur at temperatures below a certain, so-called critical temperature T cr. For example, for water the critical temperature is 647.3 K, for nitrogen 126 K, for oxygen 154.3 K. At room temperature (≈ 300 K) water can be in both liquid and gaseous states, and nitrogen and oxygen exist only in the form of gases.
Evaporation called a phase transition from a liquid to a gaseous state. From the point of view of molecular kinetic theory, evaporation is a process in which the fastest molecules fly out from the surface of a liquid, the kinetic energy of which exceeds the energy of their connection with the remaining molecules of the liquid. This leads to a decrease in the average kinetic energy of the remaining molecules, i.e., to cooling of the liquid (if there is no energy supply from surrounding bodies).
Condensation is a process reverse process evaporation. During condensation, the vapor molecules return to the liquid.
In a closed container, liquid and its vapor can be in a state dynamic equilibrium, when the number of molecules flying out of the liquid is equal to the number of molecules returning to the liquid from the vapor, i.e. when the rates of the evaporation and condensation processes are the same. Such a system is called two-phase . Vapor that is in equilibrium with its liquid is called rich.
The number of molecules emitted from a unit surface area of a liquid in one second depends on the temperature of the liquid. The number of molecules returning from vapor to liquid depends on the concentration of vapor molecules and their average speed thermal movement, which is determined by the temperature of the steam. It follows that for a given substance the concentration of vapor molecules at equilibrium of the liquid and its vapor is determined by their equilibrium temperature. The establishment of dynamic equilibrium between the processes of evaporation and condensation with increasing temperature occurs at higher concentrations of vapor molecules. Since the pressure of a gas (steam) is determined by its concentration and temperature, we can conclude: pressure saturated steam p 0 of a given substance depends only on its temperature and does not depend on volume. Therefore, isotherms of real gases on the plane ( p, V) contain horizontal sections corresponding to a two-phase system (Fig. 3.4.1).
As the temperature increases, the saturated vapor pressure and density increase, and the density of the liquid decreases due to thermal expansion. At a temperature equal to the critical temperature T kr for a given substance, the densities of vapor and liquid become the same. At T > T kr the physical differences between the liquid and its saturated vapor disappear.
If you compress unsaturated steam isothermally at T < T kr, then its pressure will increase until it becomes equal to the saturated vapor pressure. With a further decrease in volume, a liquid forms at the bottom of the vessel and a dynamic equilibrium is established between the liquid and its saturated vapor. With a decrease in volume everything most of the steam condenses, but its pressure remains unchanged (horizontal section on the isotherm). When all the vapor turns into liquid, the pressure increases sharply with a further decrease in volume due to the low compressibility of the liquid.
You can go from a gaseous state to a liquid state without passing through the two-phase region. To do this, you need to perform a process that bypasses the critical point K. One of the possible processes of this kind is shown in Fig. 1 by a broken line ABC.
Atmospheric air always contains water vapor at a certain partial pressure p, which is usually less than the saturated vapor pressure p 0 . Attitude p / p 0 expressed as a percentage is called relative humidity air.
| |
Unsaturated steam can be theoretically described using the equation of state of an ideal gas under the usual restrictions for real gases: the steam pressure should not be too high (practically p≤ (10 6 –10 7) Pa), and its temperature is higher than a certain value specific for each substance. The ideal gas laws can also be approximately applied to saturated steam, provided that for each temperature T pressure p 0 saturated steam is determined by equilibrium curvep 0 (T) for a given substance.
Pressure p 0 saturated steam increases very quickly with increasing temperature T. Addiction p 0 (T) cannot be obtained from the ideal gas laws. At a constant concentration of molecules, the gas pressure increases in direct proportion to the temperature. In saturated steam, as the temperature rises, not only the average kinetic energy of molecular motion increases, but also their concentration. Therefore, the pressure of saturated vapor with increasing temperature increases faster than the pressure of an ideal gas at a constant concentration of molecules.
Evaporation can occur not only from the surface, but also in the bulk of the liquid. Liquids always contain tiny gas bubbles. If the vapor pressure of a liquid is equal to or greater than the external pressure (i.e., the pressure of the gas in the bubbles), the liquid will evaporate into the bubbles. Bubbles filled with steam expand and float to the surface. This process is called boiling . Thus, the boiling of a liquid begins at a temperature at which the pressure of its saturated vapor becomes equal to the external pressure.
In particular, at normal atmospheric pressure, water boils at a temperature of 100 °C. This means that at this temperature the pressure of saturated water vapor is 1 atm. When ascending mountains, atmospheric pressure decreases, and therefore the boiling point of water decreases (by approximately 1 °C for every 300 meters of altitude). At an altitude of 7 km, the pressure is approximately 0.4 atm, and the boiling point drops to 70 °C.
In a hermetically sealed vessel, a liquid cannot boil, since at each temperature value an equilibrium is established between the liquid and its saturated vapor. According to the equilibrium curve p 0 (T) it is possible to determine the boiling point of a liquid at various pressures.
The picture of real gas isotherms shown in Fig. 1 describes the processes of evaporation and condensation, i.e., the phase transition between gaseous and liquid phases substances. In fact, this picture is incomplete, because any substance can go from gaseous and liquid to a solid state. At a given temperature T thermodynamic equilibrium between two phases of the same substance is possible only at a certain value of pressure in the system. The dependence of equilibrium pressure on temperature is called phase equilibrium curve . An example is the equilibrium curve p 0 (T)saturated vapor and liquid. If equilibrium curves between different phases of a given substance are plotted on a plane ( p, T), then they divide this plane into separate areas in which the substance exists in a homogeneous state of aggregation - solid, liquid or gaseous (Fig. 2). Depicted in the coordinate system ( p, T) equilibrium curves are called phase diagram .
Curve 0 T, corresponding to the equilibrium between the solid and gaseous phases, is called sublimation curve. Curve TK equilibrium between liquid and vapor is called evaporation curve, it breaks off at a critical point K. Curve TM equilibrium between a solid and a liquid is called melting curve.
Equilibrium curves converge at a point T, in which all three phases can coexist in equilibrium. This point is called triple point.
For many substances the pressure p tr at the triple point is less than 1 atm ≈ 10 5 Pa. Such substances melt when heated at atmospheric pressure. For example, the triple point of water (Fig. 3) has coordinates T tr = 273.16 K, p tr = 6.02·10 2 Pa and is used as a reference for calibrating the absolute Kelvin temperature scale.
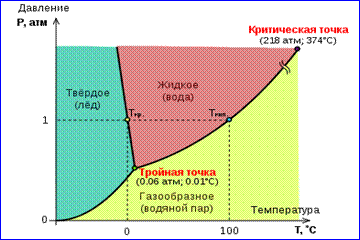 There are, however, such Fig. 3 Phase diagram of water
There are, however, such Fig. 3 Phase diagram of water
substances that have p tr
exceeds 1 atm. So for
carbon dioxide (CO 2) pressure
p tr = 5.11 atm and temperature
T tr = 216.5 K. Therefore, at atmospheric
pressure, solid carbon dioxide can
exist only at low temperatures, and in a liquid state at p= 1 atm it does not exist at all. In the solid state, in equilibrium with its vapor at atmospheric pressure, carbon dioxide is at a temperature of 173 K or –80 °C. This is a widely used “dry ice” that never melts, but only evaporates (sublimates).
