A. Rutherford's experiments
In 1911, Rutherford conducted experiments of exceptional significance that proved the existence of the atomic nucleus. To study the atom, Rutherford used its probing (bombardment) using alpha particles, which arise during the decay of radium, polonium and some other elements. Rutherford and his co-workers, in earlier experiments in 1909, established that α-particles have a positive charge equal in magnitude to twice the electron charge q =+2e, and a mass that coincides with the mass of a helium atom, that is
However, the daughter side of chemical separation is only intended to decay, since there is no means to replenish the supply of converted atoms. Rutherford and Soddy saw what appeared to be permanent activity uranium, thorium and radium are associated with half-lives that are long compared to human lives. This understanding overcame the conundrum at the heart of all previous theories; for if the total radioactivity in the Universe became less and less and tended to disappear, the law of conservation of energy would not be violated.
m A= 6.62 10 -27 kg,
which is about 7300 times the mass of an electron. It was later discovered that α particles are the nuclei of helium atoms. Rutherford bombarded the atoms of heavy elements with these particles. Electrons, due to their low mass, cannot change the trajectory of an α-particle. Their scattering (change in direction of movement) can only be caused by the positively charged part of the atom. Thus, from the scattering of α-particles, it is possible to determine the nature of the distribution of positive charge, and therefore mass, inside the atom.
They consider radioactivity to be a fundamental property of nature, suitable to join the select group of electricity, magnetism, light and gravity. The least remarkable thing about this theory, which declared that the atom was not indestructible, was the uncontroversial manner in which it was accepted. Apart from the elderly and unchanging Lord Kelvin and the constantly controversial Henry Armstrong, the theory of transformation encounters little opposition, especially chemists, although it violates the idea of \u200b\u200bthe immutability of atoms, that they are "absorbed with mother's milk", cannot refute evidence and, at best, could accept expected attitude.
It was known that alpha particles emitted by polonium fly at a speed of 1.6-107 m/s. Polonium was placed inside a lead case, along which a narrow channel was drilled. A beam of α-particles, having passed through the channel and the diaphragm, fell on the foil. Gold foil can be made extremely thin - 4-10 -7 m thick (400 gold atoms; this number can be estimated by knowing the mass, density and molar mass of gold). After the foil, the α-particles fell on a translucent screen coated with zinc sulfide. The collision of each particle with the screen was accompanied by a flash of light (scintillation) caused by fluorescence, which was observed through a microscope.
To a large extent, Rutherford spent the following years developing this rich vein of interpretation. Working with Soddy and using a new liquid air machine provided to McGill by his wealthy benefactor, he condensed the emanation at low temperatures, proving it to be a gas. Other tests convinced them that the emanation belonged to the family of noble gases discovered shortly before by Sir William Ramsay. Such work was very important because there were many radioelements, of which the chemical identity and place in the decay series were uncertain.
With a good vacuum inside the device (so that there was no scattering of particles from air molecules), in the absence of foil, a light circle of scintillations caused by a thin beam of α-particles appeared on the screen. When foil was placed in the path of the beam, the vast majority of α particles still did not deviate from their original direction, that is, they passed through the foil as if it were empty space. However, there were alpha particles that changed their path and even bounced back.
What is the logical inconsistency of the planetary model of the atom?
Helium, although not a radio element, was of particular interest because of Rutherford's belief that as a positive ion it was identical to the alpha particle. And he considered the alpha particle, which has a significant mass, to be the key in the transition from an element of one atomic weight to an element of another. He fascinated Rutherford also because he could appreciate the enormous speed and energy with which he was thrown out of the decaying atom.
Since Boltwood remained in New Haven, Connecticut, his collaboration with Rutherford was conducted by mail. This work continued from determining the amount of radium present per gram of uranium in minerals to Rutherford's proposal that if the amount and rate of formation of the final product of the series were known, the age of the mineral could be calculated. Strutt in England followed this idea, using helium found in radioactive substances; but the variable quantity of this gas which was escaped allowed only a minimal determination of age.
Marsden and Geiger, Rutherford's students and collaborators, counted more than a million scintillations and determined that approximately one in 2 thousand alpha particles was deflected at angles greater than 90°, and one in 8 thousand was deflected by 180°. It was impossible to explain this result on the basis of other models of the atom, in particular Thomson.
Calculations show that when distributed over the entire atom, a positive charge (even without taking into account electrons) cannot create a sufficiently intense electric field capable of throwing an α particle back. The electric field strength of a uniformly charged ball is maximum on the surface of the ball and decreases to zero as it approaches the center. The scattering of α-particles at large angles occurs as if the entire positive charge of the atom was concentrated in its nucleus - a region occupying a very small volume compared to the entire volume of the atom.
The bolt tree showed a universal occurrence of lead with uranium minerals; considered it the end product of the series; and, using Rutherford's value for the half-life of radium and their value for the amount of radium in the uranium graph, was able to calculate the rate of formation of lead. Age of some of his specimens rocks was more than a billion years old, providing quantitative evidence of the Earth's antiquity for the first time.
Many other problems in the field of radioactivity were pursued by Rutherford, sometimes alone, sometimes with one of the student researchers in the strong school he created. Among the projects in his laboratory were measurements of radiation energy, studies of the properties of beta and gamma rays, attempts to change decay rates under extreme temperature conditions, attempts to place actinium in a decay series, and studies of the radioactivity of the earth and atmosphere.
The probability of α-particles entering the nucleus and their deflection at large angles is very small, so for most α-particles the foil did not seem to exist.
Rutherford theoretically considered the problem of the scattering of α-particles in the Coulomb electric field of a nucleus and obtained a formula that allows one to determine the number of α-particles incident on the nucleus and the measured number of particles scattered at a certain angle. N elementary positive charges +e contained in the nucleus of the atoms of a given scattering foil. Experiments have shown that the number N equal to the serial number of the element in the periodic system of D.I. Mendeleev, that is N=Z(for gold Z= 79).
He was in great demand as a speaker and often traveled to remote areas of the United States and England to deliver a lecture, a series of talks, or a summer session course. Although he could not be denied the honor of speaking at the Royal Institution, the Baker Lecture on the Royal Society, or the Silliman Lectures at Yale, some well-wishers urged him to limit his external commitments. His time was also used in the writing of Radio Activity, the first textbook on the subject and recognized as a classic at its publication.
Thus, Rutherford's hypothesis about the concentration of a positive charge in the nucleus of an atom made it possible to establish the physical meaning of the serial number of an element in the periodic table of elements. A neutral atom must also contain Z electrons. It is significant that the number of electrons in an atom, determined by various methods, coincided with the number of elementary positive charges in the nucleus. This served as a test of the validity of the nuclear model of the atom.
So rapid was the progress of science, however, that Rutherford prepared a second edition the following year, which was 50 percent larger. Once this was done, he was faced with the task of putting the Silliman Lectures into a book. It is not surprising that he limited his writing to magazines for the next few years.
The real fallout of honor began to descend on him, continuing for the rest of his life. Rutherford thoroughly enjoyed this recognition because, although not in vain, he was fully aware of his own worth. While he was happy at McGill, Rutherford wanted to return to England, where he would be closer to the leading scientific centers peace.
B. Rutherford's nuclear model of the atom
Summarizing the results of experiments on the scattering of α-particles by gold foil, Rutherford established:
♦ atoms by their nature are largely transparent to alpha particles;
♦ deflections of α-particles at large angles are possible only if there is a very strong electric field inside the atom, created by a positive charge associated with a large mass concentrated in a very small volume.
Rutherford attracted an extraordinarily talented group of research students to Manchester who made profound contributions to physics and chemistry. Upon returning to England, Rutherford had only a few milligrams of radioactive material, which was not enough for his own research. In a generous gesture, the Austrian Academy of Sciences sent some 350 milligrams of radium chloride from the Joachimsthal uranium mines it controlled as a joint loan to Rutherford and Ramsey. Unfortunately, Ramsay wanted to retain his possession indefinitely, while both saw the wisdom of leaving the proposal undivided: until the Viennese authorities sent another comparable source of radium for Rutherford's exclusive use, he was limited to working with "draw" an emanation which Ramsay sent periodically from London.
To explain these experiments, Rutherford proposed a nuclear model of the atom: the entire positive charge and almost the entire mass of the atom (99.9%) are concentrated in the nucleus of the atom (an area with linear dimensions of 10 -15 -10 -14 m). Around the nucleus in a region with linear dimensions of ~10 -10 m (the dimensions of the atom are estimated in molecular kinetic theory), negatively charged electrons move in closed orbits, the mass of which is only 0.1% of the mass of the nucleus. Consequently, the electrons are located from the nucleus at a distance of 10,000 to 100,000 times the diameter of the nucleus, that is, the bulk of the atom is empty space.
State the main result of Rutherford's experiment
To a certain extent this determined most of Rutherford's initial research at Manchester, an extensive study of the emanation of radium: but he always found the emanation and its active decay products of the deposit to be more convenient sources than the radium itself.
The emanation could be easily purified in liquid air, and Rutherford soon determined the volume of this gas in equilibrium with one gram of radium. Cameron and, by confirming its calculated value, eliminated some doubts regarding the accuracy of the radioactive data and theory. Using Thomas Royds's copier spectroscope, Rutherford then photographed the spectrum of the emanation, unexplored since Ramsay and Colley's visual observations in this work included it in the scientific controversies he usually sought to avoid; but after Soddy left Ramsay's laboratory, the latter's contributions to radioactivity were noted for their almost uniform irregularity.
Rutherford's nuclear model of atoms resembles the solar system: in the center of the system there is a “sun” - the nucleus, and around it “planets” - electrons - move in orbits, which is why this model is called planetary. Electrons do not fall onto the nucleus because the electrical forces of attraction between the nucleus and the electrons are balanced by the centrifugal forces caused by the rotation of the electrons around the nucleus.
Although an expert in processing small quantities of rare gases, Ramsay never took the trouble to become thoroughly familiar with radioactivity techniques. Its inaccurate operation combined with strong desire gaining priority led him to quickly publish numerous results, which Rutherford and others in the field were forced to correct. Never limiting the scope of his research - he preferred to move along a broad path through radioactivity - Rutherford pursued “his” alpha particles.
These were his favorites: beta particles were too small and, being electrons, too common. Alphas, however, were massive, atomic-sized: and he could clearly imagine them emerging from their parent atoms with tremendous speed and energy. Of course, this will be the key to the physicist's classic goal: understanding the nature of matter. Until then, nothing had changed Rutherford's early belief that the alpha particle was a doubly charged helium atom, but he had failed to prove this belief. Collisional ionization, a process studied by Rutherford's former colleague at Cambridge, J.
In 1914, three years after creating the planetary model of the atom, Rutherford investigated the positive charges in the nucleus. By bombarding hydrogen atoms with electrons, he discovered that neutral atoms turned into positively charged particles. Since the hydrogen atom has one electron, Rutherford decided that the nucleus of the atom is a particle carrying an elementary positive charge +e. He called this particle proton.
Townsend, caused an increase in the charge of a single particle sufficient to give the electrometer a measurable "bump". Thus they were able to calculate, for the first time accurately and directly, the number of alpha particles emitted per second from a gram of radium. This experiment allowed Rutherford and Geiger to confirm that each alpha particle produced a weak but discrete flash when it struck a zinc sulfide fluorescent screen and therefore led directly to the widespread method of scintillation counting.
It was also the origin of the electrical and electronic particle counting methods in which Geiger was later founded. But at this time the scintillation technique, which now proved reliable, was more convenient. They measured the total charge from the radium source and divided it by the amount of alpha calculated to give the charge per particle. But Rutherford still wanted decisive, direct evidence; and here his experienced glassblower helped him.
The planetary model agrees well with experiments on α-particle scattering, but it cannot explain the stability of the atom. Consider, for example, a model of a hydrogen atom containing a proton nucleus and one electron that moves at a speed v around the nucleus in a circular orbit of radius r. The electron must fall in a spiral onto the nucleus, and the frequency of its revolution around the nucleus (and therefore the frequency of the electromagnetic waves emitted by it) must continuously change, that is, the atom is unstable, and its electromagnetic radiation must have a continuous spectrum.
Such a tube was filled with emanation and placed in a larger tube made of thicker glass. Over time, alpha particles from the decaying emanation penetrated and were trapped in the space between the inner and outer tubes: when Royds ignited the material in this space, they saw the spectrum of helium. As in Montreal, Rutherford found chemical care in Manchester to be of the highest quality. Boltwood spent a year with him, during which they more accurately determined the rate of production of helium by radium. Combining these results with the results of the counting experiments mentioned above, they obtained Avogadro's number larger than ever before.
In reality it turns out that:
a) the atom is stable;
b) an atom emits energy only under certain conditions;
c) the radiation of an atom has a line spectrum, determined by its structure.
Thus, the application of classical electrodynamics to the planetary model of the atom led to a complete contradiction with experimental facts. Overcoming the difficulties that arose required the creation of a qualitatively new quantum– atomic theories. However, despite its inconsistency, the planetary model is still accepted as an approximate and simplified picture of the atom.
There were also new researchers to the periodic table - Alexander Russell, Casimir Fajans and Georg von Hevesy, creating information and ideas on the basis of which displacement laws and the concept of isotope would be established, as well as work on decay series branching, periods of short-lived elements and other radiochemical problems .
Rutherford's greatest discovery at Manchester - indeed of his career - was the nuclear structure of the atom. In retrospect, its origins can be seen in the minor evidence of alpha particle scattering in thin metal foils or mica sheets that he noticed while at McGill, and in similar scattering by air molecules in his later electrical counting experiments with Geiger. To learn more about this scattering, both because it introduced experimental difficulties leading to less accurate results and because it was associated with the puzzling question of the nature of absorption alpha and beta in matter, Geiger made a quantitative study of this phenomenon.
MINISTRY OF HIGHER AND SECONDARY SPECIAL EDUCATION OF THE RF.
NOVOSIBIRSK STATE ARCHITECTURAL AND CONSTRUCTION UNIVERSITY
Department of Physics
ABSTRACT
Rutherford's experiments
Completed: Kuznetsov I.A. (group 226)
Checked: Berkhoer L.D.
Novosibirsk 2000
Ernest Rutherford is one of the most famous physicists of the first half of the 20th century. Once upon a time, Rutherford was the first to dissect an atom, discovering a nucleus in it. He investigated the complex phenomena occurring in this amazingly small particle of matter, and then in his laboratory he split the nuclei of atoms.
While still a 2nd year student at the university, Rutherford gave a presentation at one of the conferences on the topic “Evolution of the Elements.” Rutherford suggested that all chemical elements are complex chemical systems consisting of the same elementary particles. At that time, the atom was considered indivisible - Dalton’s theory of the indivisibility of atoms dominated in physics.
The first attempt to create an atomic model based on accumulated experimental data was made by J. J. Thomson. Electrons, as Thomson thought, are embedded in a subminiature sphere with a diameter of 10–8 cm, in which positive charges are evenly distributed. Together with negatively charged electrons, the sphere is electrically neutral. This is the atom. At that time, Rutherford, who worked in the same laboratory with Thomson, thought so too, and did not even dream that he could create a more advanced model based on new ideas.
In 1896, while studying luminescence various substances, A. Becquerel accidentally discovered that uranium salts emit without prior illumination. This radiation has great penetrating power and is capable of affecting a photographic plate wrapped in black paper. Rutherford immediately began studying Becquerelian rays. He began his research on X-rays by testing his hypothesis about the connection between X-rays and becquerelium rays. This idea came to him for a very simple reason: both of them produced ionization of the air. This idea was not successful.
But Rutherford's most important result was the discovery of -particles in the radiation emitted by uranium. Rutherford placed a uranium source in a strong magnetic field and separated the radiation into three different types. In other words, he then discovered the composition of radioactivity: alpha and beta particles and gamma rays.
Having received the particles, Rutherford immediately made the brilliant conclusion that they constituted a powerful tool for penetrating into the depths of the atom. As was later confirmed, this was absolutely correct. In subsequent works, Rutherford made extensive use of -astics as projectiles penetrating the heart of the atom - the atomic nucleus.
Rutherford discovered the emanation of thorium and proved that this radioactive gas released from thorium was a chemical element different from thorium itself. Later, he determined the atomic weight of the emanation and showed that it is a noble gas of the zero group of the D.I. Mendelev system.
Rutherford and Frederick Soddy were the first to explain radioactive decay as the spontaneous transition of one element to another. After the emanation of thorium, Rutherford discovered the emanation of radium - radon. It was clear to the scientist that radium, emitting particles, turns into a new active substance, similar to the emanation of thorium. This discovery finally confirmed the theory of radioactive decay.
At the beginning of 1903, Rutherford tried experimentally to determine the chemical composition of particles. The idea is to compare the mass of a particle with the masses of atoms of known elements. Experience allowed him to be the first to identify particles with helium atoms. This was later confirmed spectrographically.
In 1908, Rutherford began extensive experiments in the study of particles by counting them using a Geiger scintillation counter.
![]() Together with Geiger and Royds, Rutherford carried out a series of experiments confirming that -particles are nothing more than doubly ionized (i.e., having lost 2 electrons) helium atoms. This historical experience, thanks to which no one could have any doubt about the correctness of his theory of decay, consisted of the following:
Together with Geiger and Royds, Rutherford carried out a series of experiments confirming that -particles are nothing more than doubly ionized (i.e., having lost 2 electrons) helium atoms. This historical experience, thanks to which no one could have any doubt about the correctness of his theory of decay, consisted of the following:
Rutherford placed a certain amount of radon, an emanation of radium, into a sealed tube 2. The wall thickness of this tube is 0.01 mm. They are thin enough so that the particles emitted by radon can pass through them into the outer tube 3. Before the experiment, tube 3 was carefully evacuated, and helium lines could not be detected in it spectrographically. A few days later, gas accumulation was discovered in tube 3. By increasing the pressure in the device, the accumulated gas could be concentrated in tube 1. electric charge and then it turned out that spectral analysis in it shows characteristic lines of helium. There was helium in the tube. But maybe it got into tube 2 through an oversight along with radon, and from there it penetrated into tubes 3 and 1? The control experiment gave a negative answer to this question. In exactly the same device (in tube 2), Rutherford placed not radon, but pure helium. However, after a few days, no helium lines were detected in tube 1. Helium could not pass through the glass walls of tube 2 into tube 3. -particles easily passed through the glass and accumulated in tube 3, and then concentrated in tube 1, where they were subjected to spectral analysis, giving helium lines.
After this, Rutherford, together with Geiger and Marsden, conducted new series experiments. The results revolutionized physics. It was the most dramatic chapter in science of our time. Rutherford discovered the atomic nucleus and thereby founded a new and extremely important science - nuclear physics.
What kind of experiments were these? Rutherford and Geiger initially continued their observations of scintillations caused by γ-particles upon impact with a luminescent screen made of zinc sulfide. First of all, experiments led Rutherford to the conclusion that each flash (scintillation) is caused by one particle. Thus, the assumption he made earlier was justified. Rutherford wrote then that observing scintillations on a zinc sulfide screen was a very convenient way of counting particles if each particle caused a flash. Consequently, if each flare is caused by a single particle, then physicists have the opportunity to observe the behavior of individual atoms.
Rutherford and Geiger visually calculated that over the course of a second, 130,000 α-particles are emitted from an emitter of one thousandth of a gram of radium. The accuracy of the count was impeccable. Both scientists, who were later joined by Marsden, spent many hours in a darkened laboratory doing tedious scintillation calculations. Geiger said that he alone had to count a total of a million particles.
Rutherford's student Marsden began his work. He was tasked with counting particles passing through thin metal plates. These plates were placed in the device between the particle emitter and the luminescent screen.
In entrusting this work to Marsden, Rutherford did not expect to find anything interesting. Provided that Thomson's model of the atom was correct (and then there was no reason to doubt it), experiment should have shown that -particles pass freely through metallic barriers. However, something still forced Rutherford to undertake this new experiment.
Marsden was struck by the fact that the particles in this simple experiment behaved differently than they should if we accepted the model of the atom as proposed by Thomson. According to Thomson's model, the positive charge is distributed throughout the entire volume of the atom and is balanced by the negative charge of electrons, each of which has a mass much less than the mass of the particle. Therefore, even in rare cases when a particle collides with an electron that is much lighter in comparison with itself, it can only slightly deviate from its straight path. But in Marsden's experiments, the particles did not pass unhindered through the metal plate. No, some of them deviated after hitting the plate at an angle of about 150 o, i.e. almost back to the emitter. There were, however, very few such returning particles. When the experimenter blocked the path of the particles with a thicker plate, more particles deflected at large angles appeared in his field of vision. This indicated that the scattering of particles observed by Marsden did not represent some kind of surface effect, i.e. it is not connected to the surface of the plate. But Marsden could not express any thoughts about the strange behavior of the particles he saw. He reported his observations in detail to Rutherford.
Rutherford later admitted that Marsden's report had an amazing effect on him: "It was almost unbelievable, as if you had fired a fifteen-pound shell at a piece of tissue paper and the shell had bounced back and hit you."
Rutherford immediately imagined that the effect observed by Marsden could occur only in one case: if the particle, having penetrated into the atom, encountered some massive obstacle present in it and was thrown back, receiving a powerful blow upon collision.
Based on these studies, Rutherford proposed a nuclear (planetary) model of the atom. According to this model, around a positive nucleus having a charge ze (z is the serial number of the element in the periodic system, e is the elementary charge), the size is 10 -15 - 10 -14 m and the mass is almost equal to mass atom, in a region with linear dimensions of the order of 10 -10 m, electrons move in closed orbits, forming the electron shell of the atom. Since atoms are neutral, the charge of the nucleus is equal to the total charge of the electrons, i.e. z electrons must rotate around the nucleus.
For simplicity, we assume that the electron moves around the nucleus in a circular orbit of radius r. In this case, the Coulomb force of interaction between the electron and the nucleus imparts centripetal acceleration to the electron. Newton's second law for an electron moving in a circle under the influence of the Coulomb force has the form ![]() , where m e and v are the mass and speed of the electron in an orbit of radius r, and is the electrical constant.
, where m e and v are the mass and speed of the electron in an orbit of radius r, and is the electrical constant.
This equation contains two unknowns: r and v. Consequently, there are countless values of radius and corresponding values of speed (and therefore energy) that satisfy this equation. Therefore, the values of r, v (and therefore E) can change continuously, i.e. Any, and not a very specific portion of energy can be emitted. Then the spectra of atoms should be continuous. In reality, experience shows that atoms have a line spectrum. It also follows from this expression that at m the speed of electrons is m/s, and the acceleration is m/s 2. According to classical electrodynamics, accelerated electrons should radiate electromagnetic waves and as a result continuously lose energy. As a result, electrons will move closer to the nucleus and eventually fall onto it. Thus, Rutherford's atom turns out to be an unstable system, which again contradicts reality.
Attempts to build a model of the atom within the framework of classical physics did not lead to success: Thomson's model was refuted by Rutherford's experiments, while the nuclear model turned out to be electrodynamically unstable and contradicted experimental data. Overcoming the difficulties that arose required the creation of a qualitatively new – quantum – theory of the atom.
The first began in 1914 World War and Rutherford had to postpone his research for a while. But periodically, while working for the military industry, he returned to his own experiments. In his next experiments, Rutherford planned to hack the atom.
These attempts were crowned with complete and stunning success. The new rise of Rutherford's genius led to a discovery that subsequently revolutionized all science and technology of our time. The first signal was given for the beginning of the atomic age. Rutherford split the atomic nucleus.
The idea of this arose in Rutherford while observing in a cloud chamber (by that time it had already been invented and improved) and in a stintillation counter of mysterious tracks (traces), much longer than the tracks of particles, well known to him from countless experiments. He thought that there were some unknown reasons for the sharp increase in the path of the particles. Another possibility (which turned out to be correct) is that the long trails are left by other unidentified particles. The researcher was faced with the task of finding out which of the two assumptions is true.
To get an answer to his questions, Rutherford decided to perform a series of experiments on bombardment of various substances with particles. He built a device that now seems incredibly simple to us. But we must also admit that only he was most suitable for a visual solution to the problem. In it, the targets for bombardment were to be gases (i.e., light atoms), rather than the metal plates usually used by Rutherford in many of his previous experiments.
The actual device built by Rutherford, with which he was able to split the nuclei of atoms of light elements for the first time, is shown schematically in the figure.
Brass tube 6 lengths 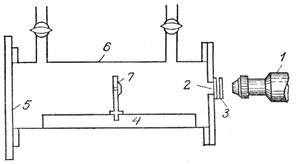 oh 20 cm with two taps is filled with gas. Inside the tube there is a disk of a radioactive emitter 7, emitting particles. This disk is mounted on a stand moving along rail 4. During the experiment, one end of the tube is covered with a frosted glass plate, and the other end with a glass plate (attached with wax). A small rectangular hole in the brass plate was closed with a silver plate 3. The silver plate had the ability to retain particles equivalent to a layer of air approximately 5 cm thick. A luminescent screen made of zinc blende was placed against the hole. To count scintillations, the researcher used telescope 1.
oh 20 cm with two taps is filled with gas. Inside the tube there is a disk of a radioactive emitter 7, emitting particles. This disk is mounted on a stand moving along rail 4. During the experiment, one end of the tube is covered with a frosted glass plate, and the other end with a glass plate (attached with wax). A small rectangular hole in the brass plate was closed with a silver plate 3. The silver plate had the ability to retain particles equivalent to a layer of air approximately 5 cm thick. A luminescent screen made of zinc blende was placed against the hole. To count scintillations, the researcher used telescope 1.
When Rutherford filled the tube with nitrogen, particles appeared in the field of view leaving a very long trail, similar to what he had already observed. Of course, Rutherford did many more experiments before coming to final conclusions. But the final conclusion was this: when β-particles collide with nitrogen nuclei, some of these nuclei are destroyed, emitting hydrogen nuclei - protons, and then the formation of an oxygen nucleus occurs.
The colossal significance of this discovery was clear from the very beginning to Rutherford himself and his collaborators. Splitting was carried out for the first time atomic nuclei. Unshakable, as it seemed before, ideas about “indecomposability” chemical elements were clearly refuted. Completely new and amazing possibilities opened up for artificially obtaining some elements from others, releasing enormous energy contained in nuclei, etc.
Continuing his research, he receives experimental confirmation of the position he had previously established - that a small number of nitrogen atoms decay during bombardment, emitting fast protons - hydrogen nuclei. In the light of later research, Rutherford wrote, “the general mechanism of this transformation is quite clear. From time to time, -particles actually penetrate into the nitrogen nucleus, momentarily forming a new nucleus such as a fluorine nucleus with a mass of 18 and a charge of 9. This nucleus, which does not exist in nature, is extremely unstable and immediately disintegrates, emitting a proton and turning into a stable nucleus oxygen with a mass of 17 ..."
As a result of lengthy experiments, Rutherford managed to cause nuclear reactions in 17 light elements.
Continuing his experiments on nuclear fission, Rutherford came to the following conclusion: although β-particles have great energy, they are still not powerful enough projectiles to penetrate the nuclei of elements. He decided to increase the energy of the particles by accelerating them in a high-voltage installation. This was the first step in the development of accelerator technology.
-
Bibliography:
1) F. Fedorov. " Chain reaction ideas", ed. “Knowledge”, M., 1975.
2) T.I. Trofimova. "Physics Course", ed. " graduate School", M., 1999.
3) "Well general physics", G.A. Zisman, O.M. Todes, ed. "Edelweiss", Kyiv, 1994.
