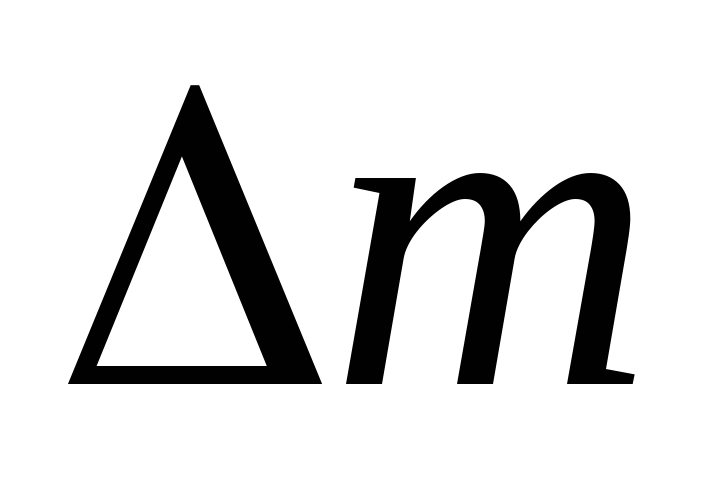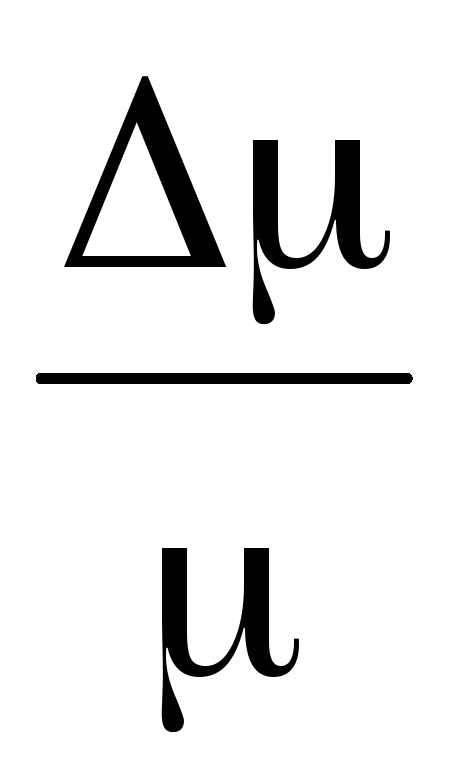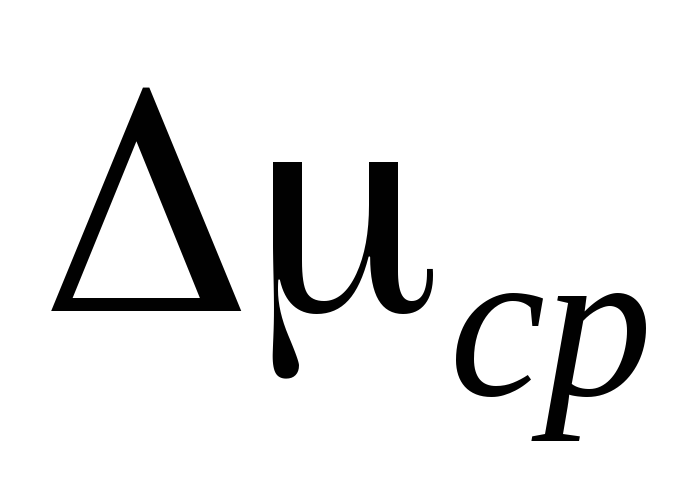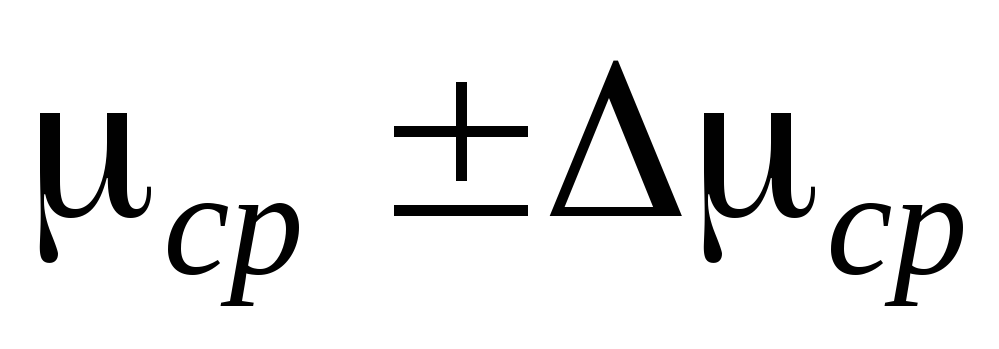Unit of measurement of molar mass: g / mol. Since the molecular masses of CO2 and H2O and the atomic mass of oxygen are respectively 44; 18 and 16a.e.m., then their molar masses are equal to: a) 44g / mol; b) 18g / mol; c) 16g / mol.
In the same way, the molecular masses of other compounds are calculated in chemical calculations. Molecular mass is a characteristic of the average mass of a molecule; it takes into account the isotopic composition of all elements that form a given chemical substance. This indicator can also be determined for a mixture of several substances whose composition is known. This law states that under the same conditions, the same number of molecules are present in the same gas volumes.
The mass of a certain known volume of gas is determined at certain pressure and temperature. This method gives fairly accurate values \u200b\u200bof molecular masses, which are sometimes even used to determine the atomic masses of chemical compounds. For a rough estimate of molecular weight, a gas is usually considered ideal, and no further corrections are made.
Air is necessary for the normal existence of living organisms on Earth. In industry and in everyday life, atmospheric oxygen is used to burn fuel to produce heat and mechanical energy in internal combustion engines. In 1754, Joseph Black experimentally proved that air is a mixture of gases and not a homogeneous substance. The first symbol is the generally accepted image of the element air. The third image is a trigram of air in the Book of Changes.
Freedom is woven from the air of man. Therefore, the symbol of air in the first place is a symbol of freedom. This is freedom, for which there are no barriers, because the air cannot be limited, you cannot catch and shape it.
Atmospheric air is a mixture of dry air and water vapor (from 0.2% to 2.6%). Thus, air can almost always be considered humid. A mechanical mixture of dry air and water vapor is called moist air or air-steam mixture. Absolute humidity is the mass of steam in 1 m3 of moist air.
When extinguishing a fire with water, both conditions are created: water cools burning objects, and its vapor impedes air access to them. The question of the composition of air in science was not immediately resolved. In 1774, the French scientist A. Lavoisier proved that air is a mixture of mainly two gases - nitrogen and oxygen. In addition, the air contains carbon monoxide (IV) and water vapor. The approximate composition of the air is shown in the table.
In an inert atmosphere of argon, electric welding of easily oxidized metals is carried out. Light bulbs fill neon, argon, krypton and xenon. You have already become familiar with the burning of substances in oxygen. During the combustion of substances in air, as a rule, the same products are formed, i.e., various oxides. Equalize the number of atoms of the elements that make up the burnt substance: C6H6 + O2-\u003e 6CO2 + 3H2O3.
This technique is used to extinguish fires in cases of burning oil and its products. The chemical composition of air has important hygienic importance, since it plays a decisive role in the implementation of the respiratory function of the body.
In residential, public and sports facilities, significant changes in the oxygen content are not observed, as the outside air enters them. With prolonged inhalation of air with a content of 1 - 1.5% of carbon dioxide, one feels worse, and with 2-2.5% pathological changes are detected.
How to find the molar mass of air
Atoms of elements are characterized by a certain (only inherent to them) mass. The values \u200b\u200bof the relative molecular mass are calculated from the values \u200b\u200bof the relative atomic mass, taking into account the number of atoms of each element in the formula unit of a complex substance. The sum of the mass fractions of the elements included in the complex substance is 1 (100%). In chemical calculations, the mass of gaseous reagents and products is often replaced by their volumes. This physical constant is the molar volume of the gas under normal conditions.
They are based on the laws of conservation of mass, constancy of composition, multiple relationships, as well as gas laws - volumetric relations and Avogadro. In production on this basis, material balances are calculated. The law is always true for gaseous and liquid substances. The law of multiple relations, like the law of constancy of composition, is not universal and also not fair for substances in the solid state. For example, in the interaction of 2 volumes of hydrogen and 1 volume of oxygen, 2 volumes of water vapor are formed. These numbers coincide with stoichiometric coefficients in the reaction equation.
The relative atomic masses of known elements are given in the table “Periodic table of elements D.I. Mendeleev. " The amount of substance B is a physical quantity that indicates the number of formula units of a substance relative to the Avogadro constant. The Avogadro constant, in turn, shows the number of atoms contained in 12 g of the carbon isotope 126C, or the number of atomic mass units in 1 g of substance.
10. Determination of molecular weights of substances in a gaseous state.
In fact, for the carbon isotope 126С Ar \u003d 12, and the molar mass of atoms (by the definition of the term “mol”) is 12 g / mol. Under normal conditions (101.325 kPa; 273 K), the molar volume of any gas is 22.4 l / mol (more precisely, Vn \u003d 22.4 l / mol). For non-ideal gases, called real, the molar volumes are different and slightly different from the exact value. If the volume and pressure of the gas are expressed in other units of measurement, then the value of the gas constant in the Clapeyron-Mendeleev equation will take a different value.
Therefore, they are used for illuminated signs and in lighthouses. The burning of substances in the air. Determination of the molar masses of substances in a gaseous state. According to the law, Avogadroravnyh volumes of gases taken at the same temperature and the same pressure contain an equal number of molecules. The molecular mass of air, like other gases, can be found using the Avogadro law.
Page 1
The molecular weight of the air is calculated taking into account the percentage of various components. By the mass of an air molecule is meant the average value of the masses of the molecules contained in the air, taking into account their relative concentration.
The molecular weight of the air is calculated taking into account the percentage of various components. By the mass of an air molecule is meant the average value of the masses of the molecules contained in the air, taking into account their relative concentration.
TO; M is the molecular weight of air; rzab - air pressure on the side.
Rvzh - the same, above the surface of the evaporating liquid, kg / m3, Мв - molecular weight of air, equal to 29; MP - the molecular weight of the vapor of the evaporating liquid.
So, to calculate the number of air molecules in the Earth’s atmosphere, it’s enough to know only the air pressure at sea level, the molecular weight of the air, the radius of the Earth and the acceleration of gravity g at its surface. The answer does not include the height of the atmosphere, it is only important that it be small in comparison with the radius of the Earth.
| Characteristics of the hydrocarbon components of natural gas. | Calculation of pseudocritical temperature and pressure of natural gas. |
Therefore, in order to determine the molecular weight of a gas, it is necessary to multiply its specific gravity (adopted for air for 1) by the molecular weight of air.
Ah, cm, for 1 sec with a pressure drop on both sides of the septum Ar, dyne / cm2; M is the molecular weight of air, g / mol; R is the universal gas constant, erg / mol is deg.
Based on the laws of ideal gases, it can be shown that the specific gravity of a gas is also equal to the ratio of the molecular weight of the gas to the molecular weight of air.
However, there may also be a mismatch between the vapor permeability of the material and the air permeability of the design of the fence made of the same material. This happens due to the inevitable presence of leaks and gaps in the structure, which significantly increase the permeability of the fence, and also because the molecular weight of air and water vapor are not the same.
To characterize natural gases, its specific gravity is widely used. Relative gas density is expressed as the ratio of gas density at atmospheric pressure and standard temperature to air density at the same pressure and temperature. Since at atmospheric pressure and a certain temperature the densities of gases are directly proportional to their molecular weights, the relative density of the gas can be represented as the ratio of the molecular weight of the gas to the molecular weight of air. The relative density of natural gases varies from 0 6 to 1 1 depending on the relative concentration of heavier hydrocarbons in the gas.
In carbon dioxide plants, the production of dry ice also has to remove air from the system. In addition to the previously mentioned paths of air penetration, in systems for the production of dry ice in ice generators, air systematically enters the system when removing blocks of ice from the ice generators. After the ice block falls out, the volume of the ice maker is filled with air, which, when the ice maker is turned on, is sucked off by the compressor and, together with carbon dioxide, enters the condenser. Due to the fact that the molecular weight of carbon dioxide is greater than the molecular weight of air, the nature of the lines of the graph pa / (ga) is similar to that for freons. But since the molecular weights of carbon dioxide and air do not differ much from freons, the lines of the graph in a significant area are close to straight lines. Nomogram for carbon dioxide, constructed by R. R. Skvarchenko (VNIIH), is shown in FIG.
In carbon dioxide plants, the production of dry ice also has to remove air from the system. In addition to the previously mentioned paths of air penetration, in systems for the production of dry ice in ice generators, air systematically enters the system when removing blocks of ice from the ice generators. After the ice block falls out, the volume of the ice maker is filled with air, which, when the ice maker is turned on, is sucked off by the compressor and, together with carbon dioxide, enters the condenser. Due to the fact that the molecular weight of carbon dioxide is greater than the molecular weight of air, the nature of the lines of the graph f (ga) is similar to that for freons. But since the molecular weights of carbon dioxide and air do not differ much from freons, the lines of the graph in a significant area are close to straight lines.
Murom Institute (branch)
federal state budgetary educational institution
higher vocational education
"Vladimir State University
named after Alexander Grigoryevich and
Nikolai Grigorievich Stoletovs
Department: "FPM"
Discipline: Physics
Laboratory work No. 6.03
Approved on methodological
seminar of the department of FPM
Head Chair ____________
Laboratory work No. 6.03
DETERMINATION OF MOLECULAR AIR MASS
Objective- get acquainted with one of the methods for determining the molecular weight of a gas and measure the molecular weight of air.
Devices and accessories: air cylinder, weighing machine or technical balance, pressure gauge, balance, vacuum pump.
SAFETY
Handle the glass bottle in the canvas bag carefully.
THEORETICAL INFORMATION
Molecular mass is the ratio of the mass of a molecule of a given substance to 1/12 of the mass of a carbon atom C.
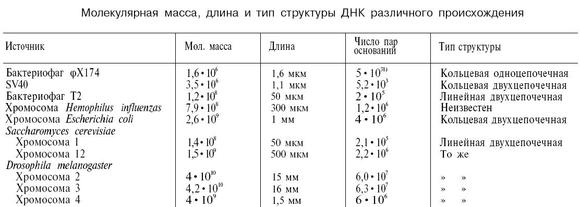
Molecular mass, by definition, can be represented as the sum of the atomic masses of the elements that make up the molecule
=
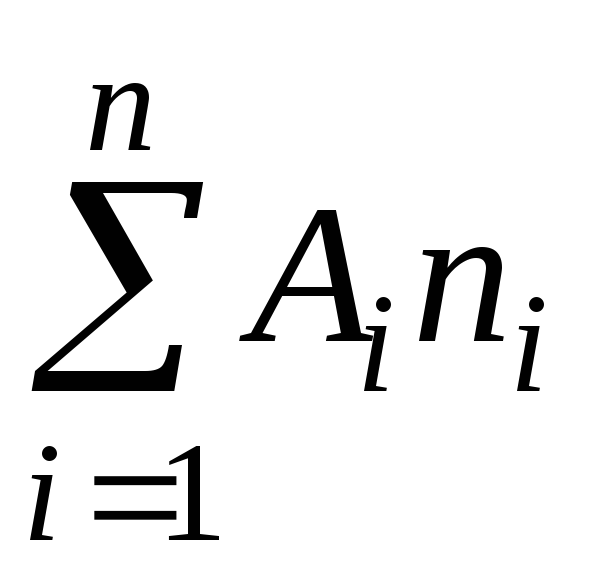 (1)
(1)
where A is the atomic mass th an element included in the composition of the molecule;
n is the number of atoms.
Molecular mass determination methods are divided into two groups — absolute and statistical. Absolute methods that give the "true" value of molecular weight include mass spectroscopy. Other methods give only the average value of molecular weight.
The determination of the molecular mass of gases is based on the gas equation of state
PV \u003d 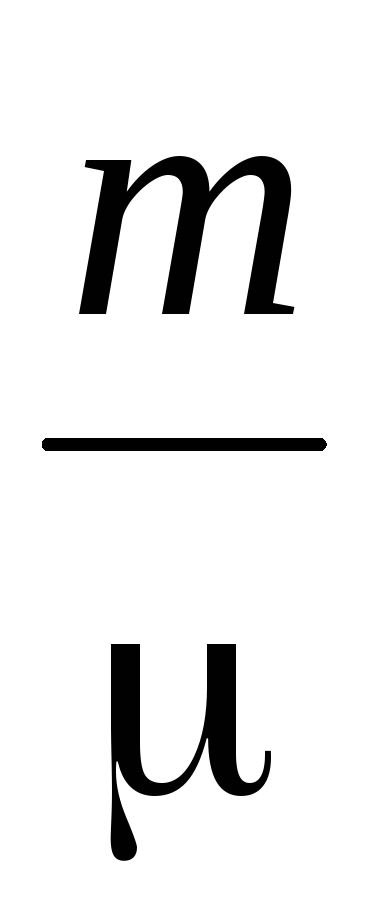 RT (2)
RT (2)
where P is the gas pressure
m, V - its mass and volume,
T - absolute temperature
R is the universal gas constant,
is the average molecular weight.
Equation 2 is valid only for an ideal gas. An ideal gas is such a gas, between the molecules of which there are no forces of interaction (attraction and repulsion). The molecules of an ideal gas are represented in the form of elastic balls of infinitely small size. Real gases have interaction forces between molecules, and not always molecules can be considered as elastic balls of vanishingly small sizes, so real gases depart from the law (2).
However, at not too high pressures, when gas molecules can travel large distances freely before a collision, the interaction of molecules can be neglected, the size of the molecules can also be neglected (when the gas volume is large enough), then the real gas will be close to ideal and equation (2) can to be applied. At atmospheric pressure and room temperature, many gases (nitrogen, hydrogen, helium, oxygen, air, etc.) can be considered an ideal gas with a fairly good approximation.
An important characteristic of molecular motion is the mean free path. The molecules in the gas are in a state of continuous and chaotic motion, collide with each other, and some path проходят passes freely between collisions. The length of this path between the two collisions is different, but due to the large number of molecules and the randomness of their motion, we can talk about the average mean free path of the molecules. The average mean free path of molecules can be determined by the formula
 =
=
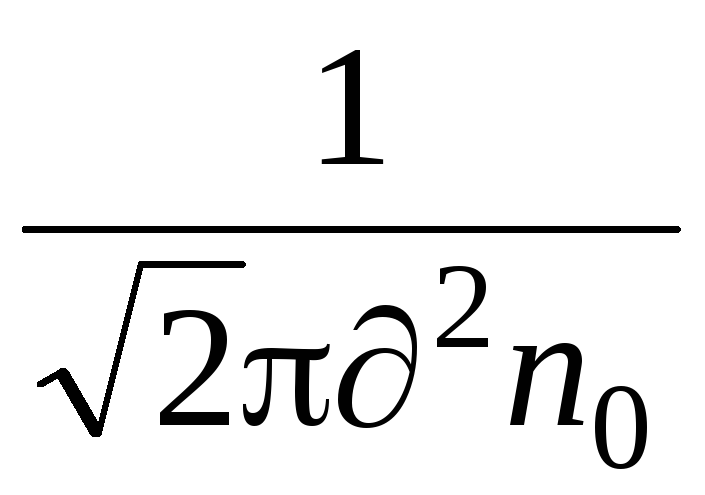 ,
,
where is the effective diameter of the molecule (for air, \u003d 0.27 · 10 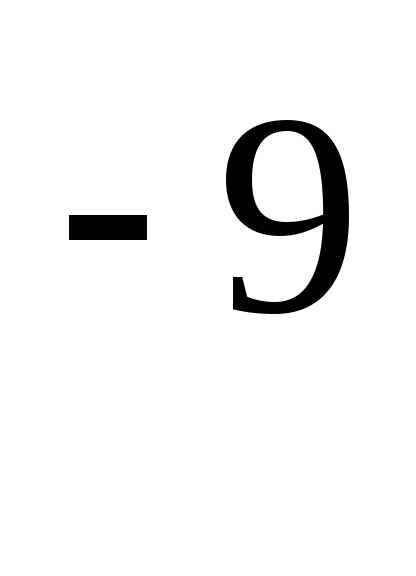 m)
m)
n 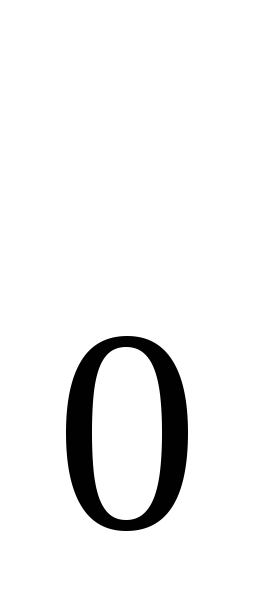 - the number of molecules per unit volume.
- the number of molecules per unit volume.
n  =
=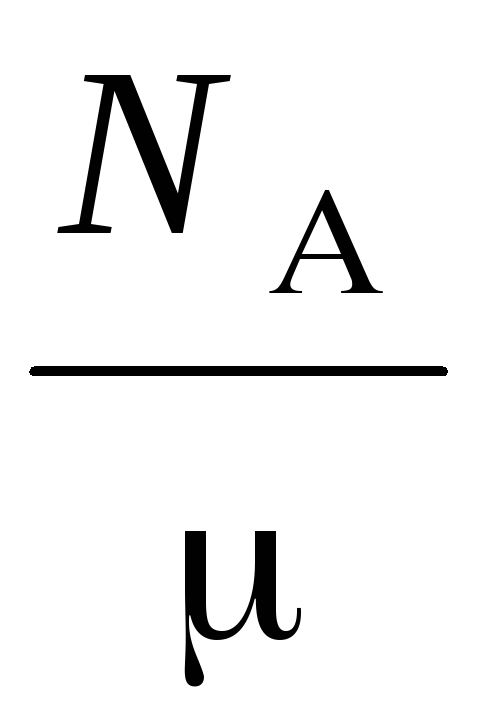
 ,
,
then given this formula , for has the form:
=
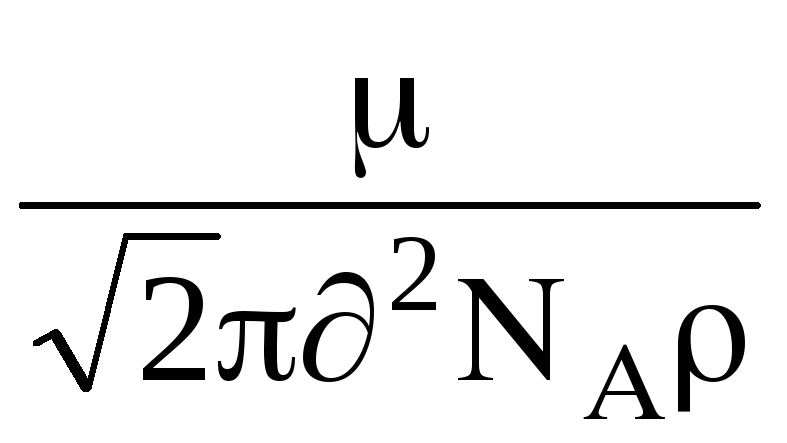 ,
,
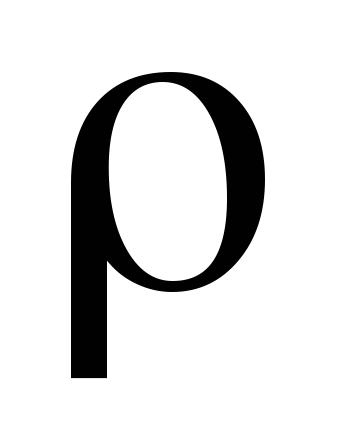
where 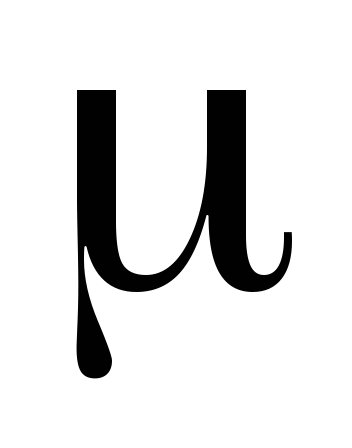 - molecular mass of gas, N
- molecular mass of gas, N 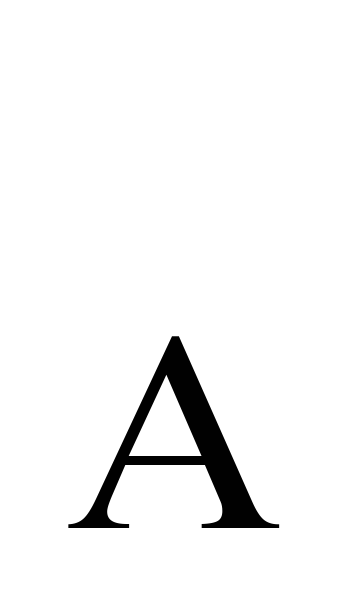 - Avogadro number, - gas density.
- Avogadro number, - gas density.
Let the air in the open cylinder take up volume Vits mass tatmospheric pressure P; pump out air from the cylinder to P  . Now the mass of air in the cylinder will be m
. Now the mass of air in the cylinder will be m  . For these two states, we write equation (2)
. For these two states, we write equation (2)
PV \u003d 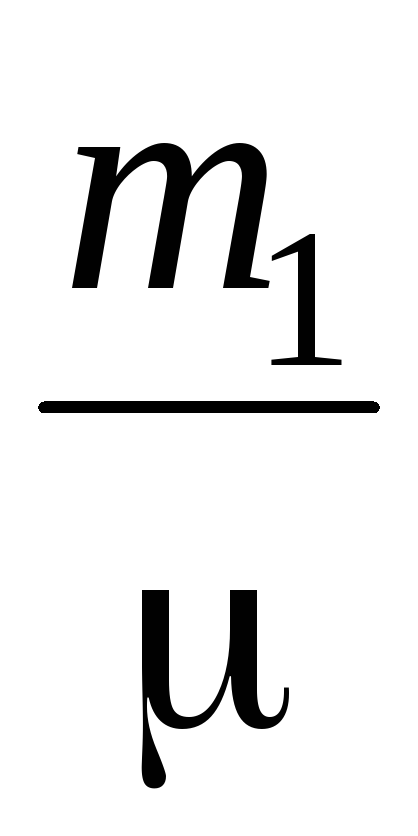 RT (3)
RT (3)
P  V \u003d
V \u003d 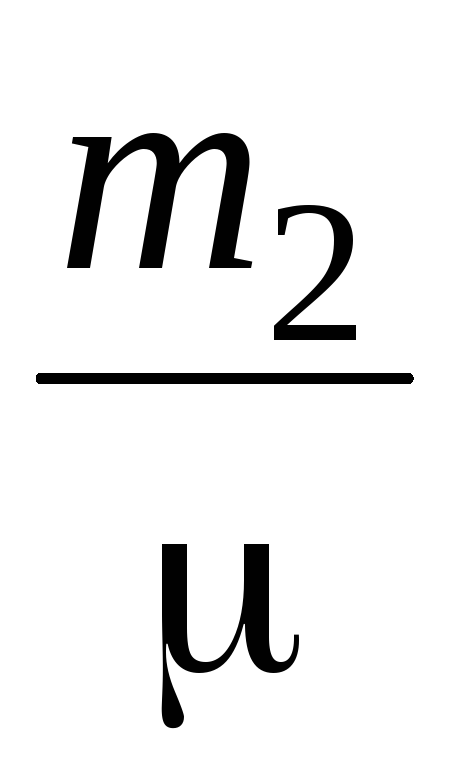 RT (4)
RT (4)
Subtracting from (3) (4), we express .
=
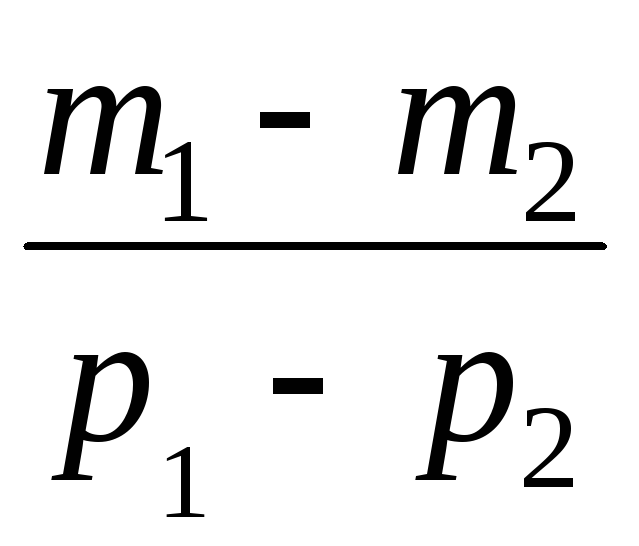
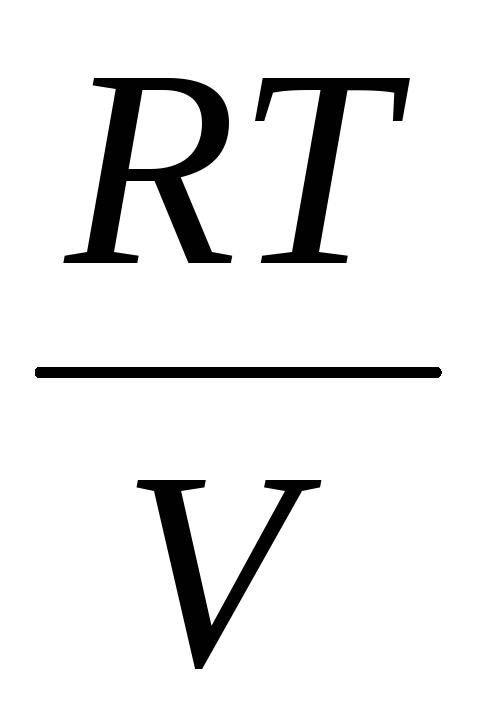 =
=
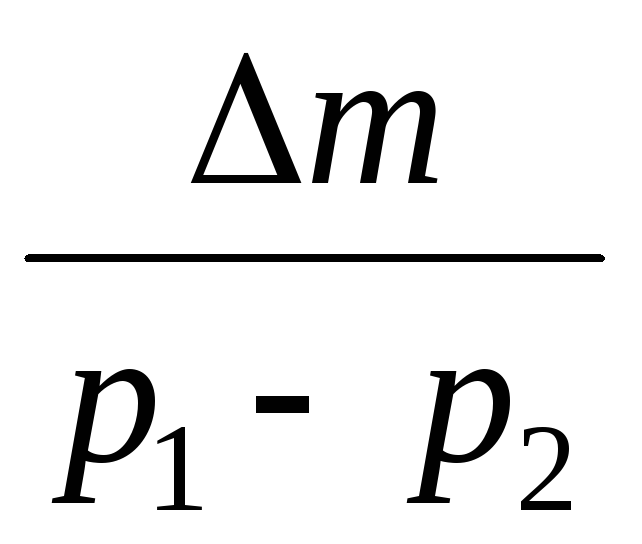
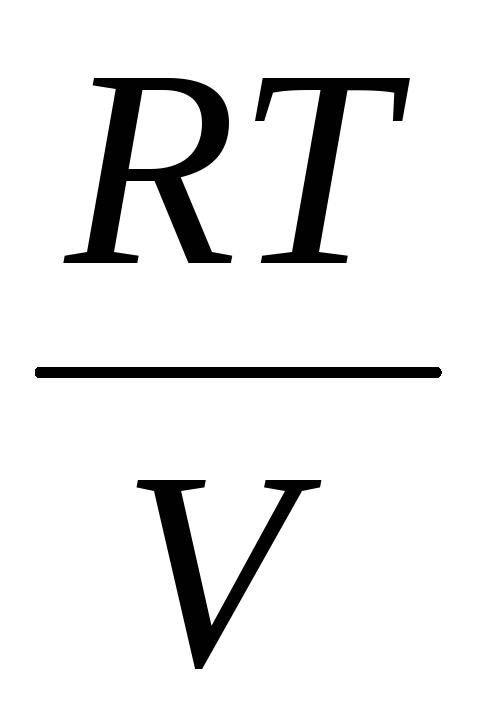 (5)
(5)
Thus, knowing the change in mass with changes in pressure, we can find the molecular mass of air by the formula (5).
INSTALLATION DESCRIPTION
The general view of the installation is shown in Fig. 1. The installation consists of a vacuum pump (1) , valve (2) (with the help of valve 2, the system is disconnected from the vacuum pump), vacuum gauge (3). The rotation of the pointer of the vacuum gauge is proportional to the vacuum achieved in the system, i.e. the difference between atmospheric pressure and air pressure in the installation. The zero value on the gauge scale corresponds to the atmospheric pressure in the installation. The device starts to show only when pumping air from the system, i.e. at air pressure in the installation below atmospheric.
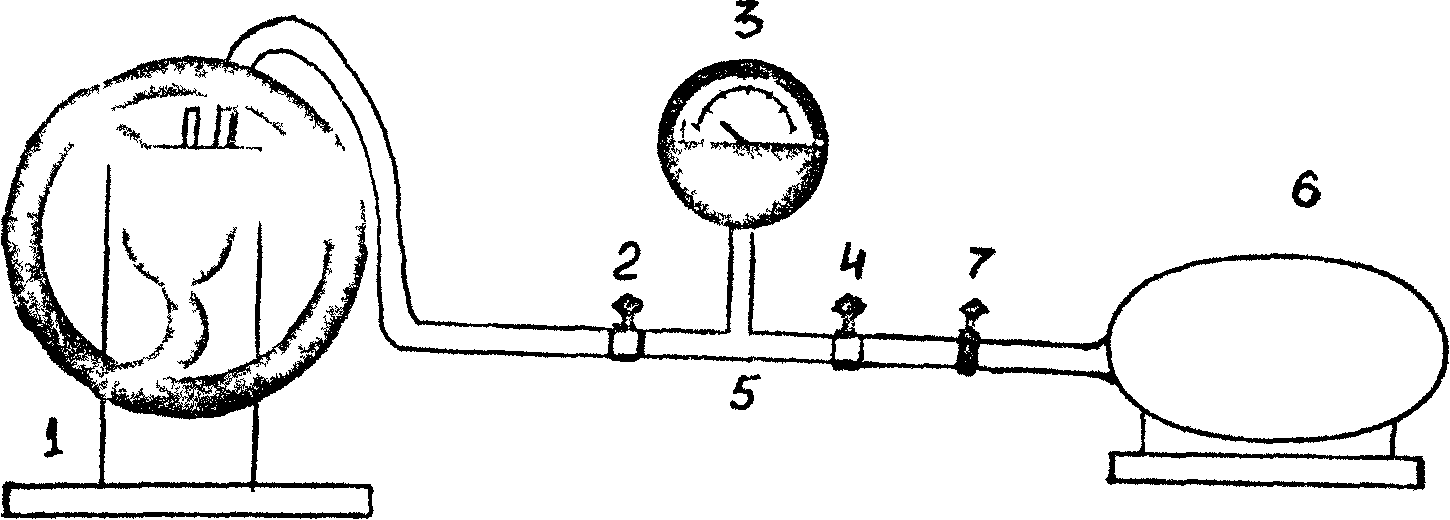
Using a crane 4, the system is connected to the atmosphere. In fig. 1 shows a vacuum pipe (5), a removable cylinder (6) (volume I/ 1225 ml) with a rubber tube and a clamp (7), which serves to disconnect the cylinder 6 from the atmosphere when weighing the cylinder.
COMPLETING OF THE WORK
Exercise 1. Determination of the molecular mass of air.
1. Open the taps 2 and 4, clamp 7 and disconnect the cylinder 6 from the installation. Weigh the balloon 6 together with the rubber tube and clamp 7 and record the measurement results in table I. Weighing must be carried out with a fairly high degree of accuracy, and this operation should be especially noted.
2. Connect the cylinder 6 to the installation and pump out the air from the cylinder so that the pressure change is 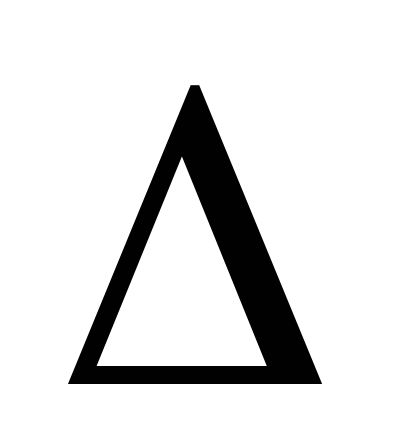 P \u003d 0.1
P \u003d 0.1 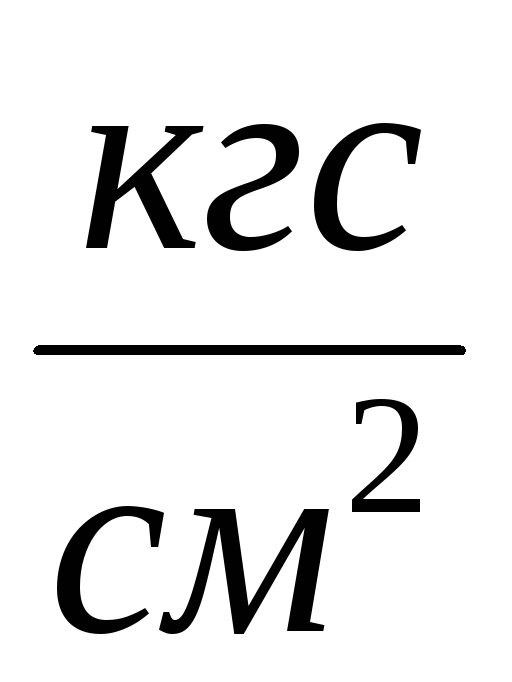 . Close the tap 2 and enter
. Close the tap 2 and enter  P to the measurement table. Close the valve 4, press 7 and disconnect the cylinder 6 from the installation and weigh it. The measurement results are entered in the table.
P to the measurement table. Close the valve 4, press 7 and disconnect the cylinder 6 from the installation and weigh it. The measurement results are entered in the table.
3. Open clamp 7, smoothly open taps 2 and 4 and step 2 repeat 4 more times for other values  P.
P.
4. Based on the measurement results, we calculate air and evaluate the measurement error.
Task 2. Determination of air density.
We determine the air density using the Mendeleev-Klaiperon equation for ideal gases.
PV \u003d 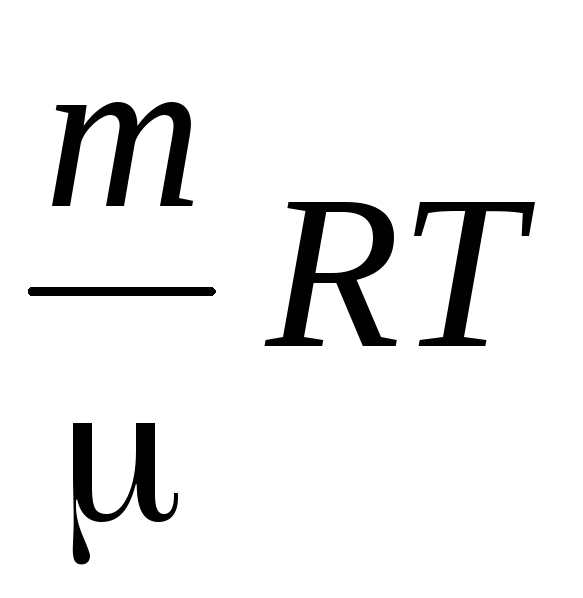 .
.
From this equation it follows that, since 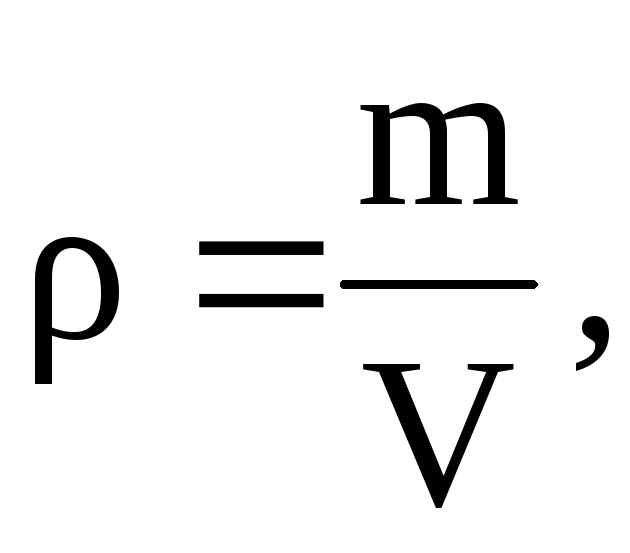 then
then
=
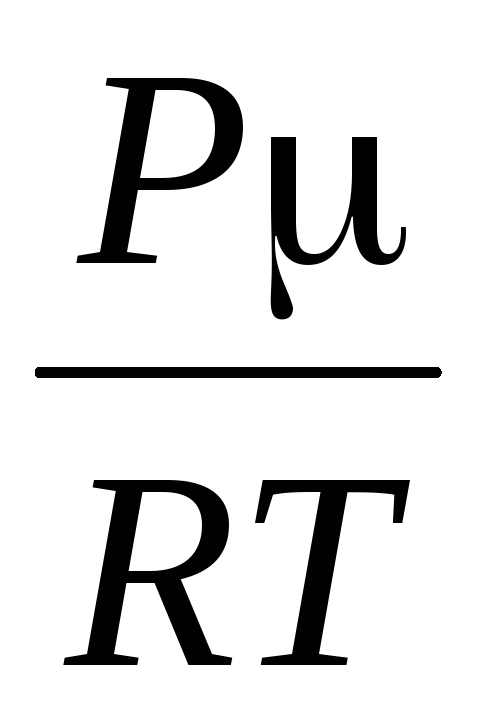 ,
,
where R \u003d 8.31 * 10 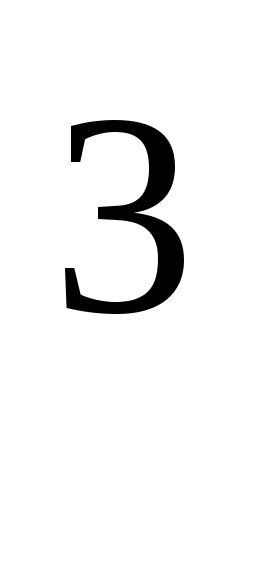 J / (kmol · K), universal gas constant.
J / (kmol · K), universal gas constant.
First, we determine the air density in the cylinder before evacuation, assuming that the pressure P \u003d P  equal to atmospheric pressure (P
equal to atmospheric pressure (P  \u003d 101 kPa). Then we determine the density of air at various
\u003d 101 kPa). Then we determine the density of air at various  P taken from the measurement table, assuming that the pressure in the flask is P \u003d P
P taken from the measurement table, assuming that the pressure in the flask is P \u003d P  -
- P.
P.
Based on the calculation results, construct a graph of the dependence of density on pressure P: 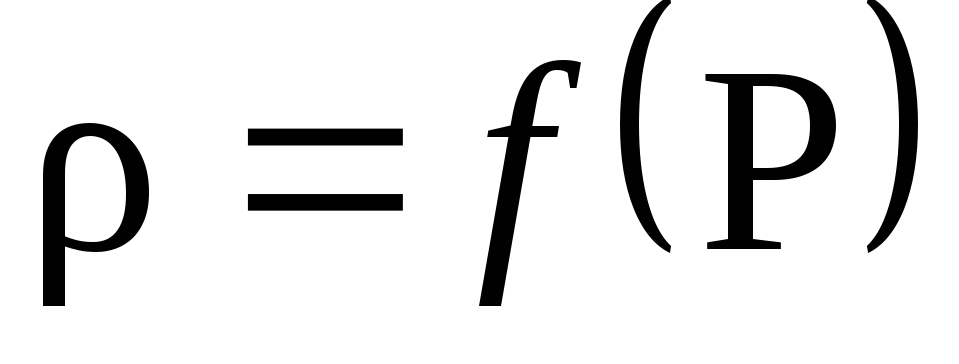
TABLE OF MEASUREMENTS
|
m |
|
|
|
|
|
| ||||||
Task 3. According to the calculated calculations , determine the mean free path and plot the dependence of on : 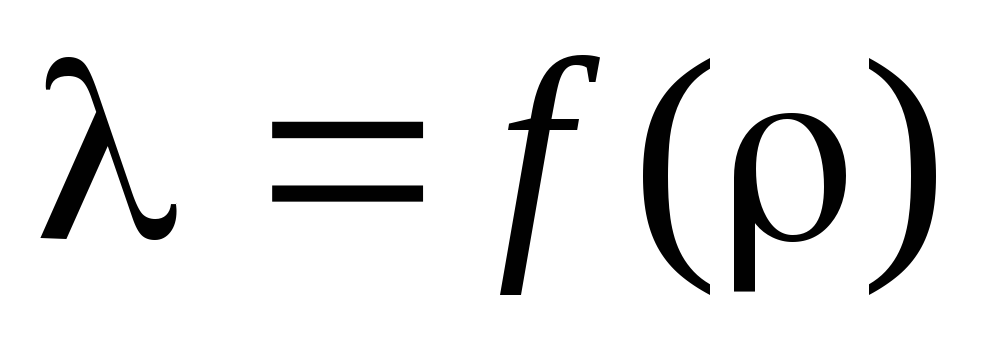 .
.
CONTROL QUESTIONS
1. List the main points of the molecular-kinetic theory of ideal gases.
2. What physical quantities are called gas state parameters, give their definition.
3. Formulate the laws of ideal gases.
4. Under what conditions does the gas obey the laws of an ideal gas?
5. Under what conditions is the Clapeyron-Mendeleev equation applicable to gases.
6. What is molecular weight, what does molecular weight depend on.
7. Density of air, what does it depend on?
8. The mean free path of gas molecules and the effective diameter.
9. Derive the formulas for calculating and .
10. What measurements should be taken to calculate the molecular weight and air density .
List of references
1. Saveliev I.V. General physics course. M .: Nauka, 1970.V.1, § 98.
2. A.A. Detlaf B.M. Yavorsky. Physics course. M.: Higher School Publishing House, 1973, pp. 175-179.