We are surrounded by various objects. We can see that they are either solids, liquids or gases. A lot of questions arise about everything that surrounds us. Provides answers to many questions molecular kinetic theory.
Molecular kinetic theory is a set of views used to describe the observable and measurable properties of a substance based on the study of the properties of atoms and molecules of a given substance, their interaction and movement.
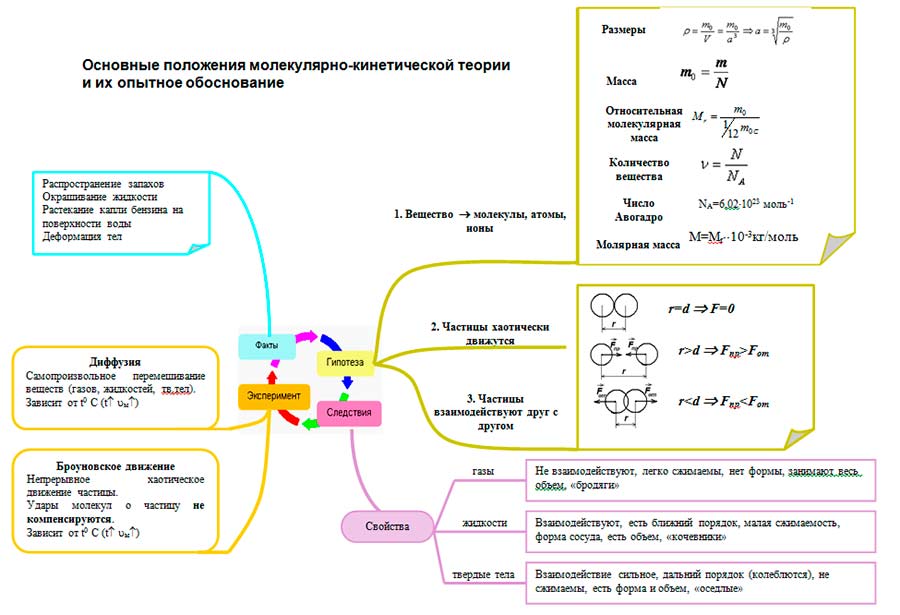
Basic principles of molecular kinetic theory
All bodies consist of particles - atoms, molecules, ions.
All particles are in continuous chaotic thermal motion.
Between the particles of any body there are forces of interaction - attraction and repulsion.
Thus, in molecular kinetic theory, the object of study is a system consisting of large quantity particles – macrosystem. The laws of mechanics are not applicable to explain the behavior of such a system. Therefore, the main research method is statistical method studying the properties of matter.
To explain and predict phenomena it is important to know main characteristics of molecules:
- Dimensions
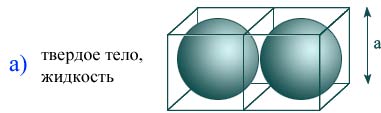
An estimate of the size of a molecule can be made as the size of the cube a that contains one molecule, based on the density of the solid or liquid and the mass of one molecule:
- Mass of molecules
Substance mass ratio m to the number of molecules N in this substance:
- Relative molecular weight
The ratio of the mass of a molecule (or atom) of a given substance to 1/12 of the mass of a carbon atom:
- Quantity of substance
The amount of substance is equal to the ratio of the number of particles N in a body (atoms - in an atomic substance, molecules - in a molecular substance) to the number of molecules in one mole of a substance NA:

- Avogadro's constant
The number of molecules contained in 1 mole of a substance.
- Molar mass
The molar mass of a substance is the mass of a substance taken in an amount of 1 mole.
IN International system units molar mass substances is expressed in kg/mol.
- Interaction (quantitatively based on experiments)
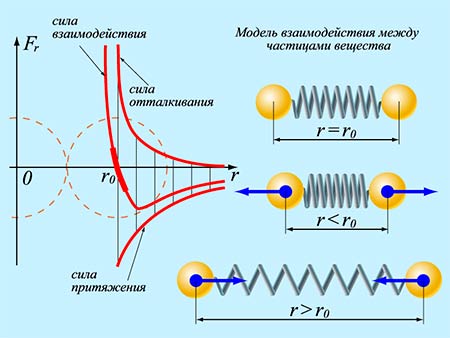
The interaction of molecules is characterized by both attraction and repulsion: at distances r
The molecular kinetic theory makes it possible to understand why a substance can exist in gaseous, liquid and solid states. From the point of view of MCT, states of aggregation differ according to the value of the average distance between molecules and the nature of the movement of molecules relative to each other.
The basic provisions of the molecular kinetic theory have been repeatedly confirmed by various physical experiments. For example, research:
A) Diffusion
B) Brownian motion
Brief summary
Molecular kinetic theory explains the structure and properties of bodies based on the movement and interaction of atoms, molecules and ions. MCT is based on three positions, which are fully confirmed experimentally and theoretically:
1) all bodies consist of particles - molecules, atoms, ions;
2) particles are in continuous chaotic thermal motion;
3) between particles of any body there are forces of interaction - attraction and repulsion.
The molecular structure of a substance is confirmed by direct observation of molecules in electron microscopes, as well as by the dissolution of solids in liquids, the compressibility and permeability of the substance. Thermal motion – Brownian motion and diffusion. The presence of intermolecular interaction with the strength and elasticity of solids and the surface tension of liquids.
Basic notes for the lesson:
Questions for self-control in the block “Basic principles of molecular kinetic theory and their experimental justification”
- Formulate the main provisions of the molecular kinetic theory.
- What observations and experiments confirm the main provisions of the molecular kinetic theory?
- What is a molecule? atom?
- What is called relative molecular weight? What formula expresses this concept?
- What is the quantity of a substance called? What formula expresses this concept? What is the unit of quantity of a substance?
- What is Avogadro's constant called?
- What is the molar mass of a substance? What formula expresses the meaning of this concept? What is the unit of molar mass?
- What is the nature of intermolecular forces?
- What properties do molecular interaction forces have?
- How do interaction forces depend on the distance between them?
- Describe the nature of molecular motion in gases, liquids and solids.
- What is the nature of particle packing in gases, liquids and solids?
- What is the average distance between molecules for gases, liquids and solids?
- List the basic properties of gases, liquids, and solids.
- What is called Brownian motion?
- What does Brownian motion indicate?
- What is diffusion called? Give examples of diffusion in gases, liquids and solids.
- 18. How does the rate of diffusion depend on the temperature of bodies?
DEFINITION
Atom - smallest particle given chemical element. All atoms existing in nature are represented in Mendeleev's periodic system of elements.
Atoms are combined into a molecule by chemical bonds based on electrical interaction. The number of atoms in a molecule can vary. A molecule can consist of one, two, three, or even several hundred atoms.
DEFINITION
Molecule- the smallest particle of a given substance that has its chemical properties.
Molecular kinetic theory- the doctrine of the structure and properties of matter based on ideas about the existence of atoms and molecules.
The founder of the molecular kinetic theory is M.V. Lomonosov (1711-1765), who formulated its main principles and applied them to explain various thermal phenomena.
Basic principles of molecular kinetic theory
Main provisions of the ICT:
- all bodies in nature consist of tiny particles (atoms and molecules);
- particles are in continuous chaotic movement, which is called thermal;
- particles interact with each other: forces of attraction and repulsion act between particles, which depend on the distance between the particles.
The molecular kinetic theory is confirmed by many phenomena.
The mixing of various liquids and the dissolution of solids in liquids is explained by the mixing of molecules of various kinds. In this case, the volume of the mixture may differ from the total volume of its components. which indicates different sizes of molecular compounds.
DEFINITION
Diffusion- the phenomenon of penetration of two or more contacting substances into each other.
Diffusion occurs most intensely in gases. The spread of odors is due to diffusion. Diffusion indicates that molecules are in constant chaotic motion. Also, the phenomenon of diffusion indicates that there are gaps between the molecules, i.e. matter is discrete.
DEFINITION
Brownian motion - thermal movement tiny microscopic particles suspended in a liquid or gas.
This phenomenon was first observed by the English botanist R. Brown in 1827. Observing flower pollen suspended in water through a microscope, he saw that each pollen particle made rapid, random movements, moving over a certain distance. As a result of individual movements, each pollen particle moved along a zigzag trajectory (Fig. 1, a).
Fig.1. Brownian motion: a) trajectories of movement of individual particles suspended in a liquid; b) transfer of momentum from liquid molecules to a suspended particle.
Further studies of Brownian motion in various liquids and with various solid particles showed that this motion becomes more intense the more smaller size s particles and the higher the temperature of the experiment. This movement never stops and does not depend on any external reasons.
R. Brown was unable to provide an explanation for the observed phenomenon. The theory of Brownian motion was constructed by A. Einstein in 1905 and received experimental confirmation in the experiments of the French physicist J. Perrin (1900-1911).
Liquid molecules, which are in constant chaotic motion when colliding with a suspended particle, transfer some momentum to it (Fig. 1, b). In the case of a large particle, the number of molecules incident on it from all sides is large, their impacts are compensated at each moment of time, and the particle remains practically motionless. If the particle size is very small, then the impacts of the molecules are not compensated - more molecules can hit it on one side than on the other, as a result of which the particle begins to move. It is precisely this kind of movement that Brownian particles perform under the influence of random impacts of molecules. Although Brownian particles have a mass billions of times greater than the mass of individual molecules and move at very low speeds (compared to the speeds of molecules), their movement can still be observed through a microscope.
Examples of problem solving
EXAMPLE 1
EXAMPLE 2
1.What does it study? Molecular physics? What is MKT? 1)Molecular physics– a branch of physics that studies the physical properties of substances in various states of aggregation based on consideration of their molecular (microscopic) structure. Problems of molecular physics are solved by the methods of statistical mechanics, thermodynamics and physical kinetics; they are associated with the study of the movement and interaction of particles (atoms, molecules, ions) that make up physical bodies. 2) Molecular kinetic theory called the doctrine of the structure and properties of matter based on the idea of the existence of atoms and molecules as the smallest particles chemical substances. MCT explains the structure and properties of macroscopic bodies as a result of the interaction of a large number of atoms, molecules or ions of which they consist.
2.Formulate the main principles of MKT of a substance?The molecular kinetic theory is based on three main principles:
1) All substances - liquid, solid and gaseous - are formed from the smallest particles - molecules, which themselves consist of atoms ("elementary molecules"). all bodies consist of particles whose size can be neglected: atoms, molecules and ions; The molecules of a chemical substance can be simple or complex, i.e. consist of one or more atoms. Molecules and atoms are electrically neutral particles. Under certain conditions, molecules and atoms can acquire additional electrical charge and become positive or negative ions.
2) Atoms and molecules are in continuous chaotic motion.
3) Particles interact with each other through absolutely elastic collisions.
3.What experiments confirm the main provisions of MCT? 4.What is Brownian motion? Cause?Brownian motion- random movement of microscopic visible particles suspended in a liquid or gas solid, caused by the thermal movement of particles of a liquid or gas. Brownian motion occurs due to the fact that all liquids and gases consist of atoms or molecules - tiny particles that are in constant chaotic thermal motion, and therefore continuously push the Brownian particle from different directions. It was found that large particles with sizes greater than 5 µm practically do not participate in Brownian motion (they are stationary or sediment), smaller particles (less than 3 µm) move forward along very complex trajectories or rotate.
5.What is diffusion? Examples of using?Diffusion- the process of transferring matter or energy from an area of high concentration to an area of low concentration. An example of diffusion is the mixing of gases (for example, the spread of odors) or liquids (if ink is dropped into water, the liquid will become uniformly colored after some time). Another example is associated with a solid: atoms of contacting metals mix at the contact boundary.
6.What is a mole of a substance? Mole- a unit of measurement of the quantity of a substance in the International System of Units (SI), one of the seven basic SI units. The mole was adopted as the SI base unit by the XIV General Conference on Weights and Measures in 1971.
Mole of substance- this is the amount of it that weighs in grams the same as a molecule of a substance weighs in relative atomic units, which contains 6.02 * 10^23 structural units.
Any substance is considered by physics as a collection of smallest particles: atoms, molecules and ions. All these particles are in continuous chaotic motion and interact with each other through elastic collisions.
Atomic theory is the basis of molecular kinetic theory
Democritus
Molecular kinetic theory originated in Ancient Greece approximately 2500 years ago. Its foundation is considered atomic hypothesis , whose authors were ancient Greek philosopher Leucippus and his student ancient Greek scientist Democritus from the city of Abdera.

Leucippus
Leucippus and Democritus assumed that all material things consist of indivisible tiny particles called atoms (from Greekἄτομος - indivisible). And the space between the atoms is filled with emptiness. All atoms have size and shape and are capable of movement. Proponents of this theory in the Middle Ages were Giordano Bruno, Galileo, Isaac Beckman and other scientists. The foundations of molecular kinetic theory were laid in the work “Hydrodynamics”, published in 1738. Its author was a Swiss physicist, mechanic and mathematician Daniel Bernoulli.
Basic principles of molecular kinetic theory

Mikhail Vasilievich Lomonosov
The closest thing to modern physics was the theory atomic structure substance, which was developed by the great Russian scientist in the 18th century Mikhail Vasilievich Lomonosov. He argued that all substances are composed of molecules which he called corpuscles . And corpuscles, in turn, consist of atoms . Lomonosov's theory was called corpuscular .
But as it turns out, the atom is dividing. It consists of a positively charged nucleus and negative electrons. But in general it is electrically neutral.
Modern science calls atom the smallest part of a chemical element that is the carrier of its basic properties. Connected by interatomic bonds, atoms form molecules. A molecule can contain one or more atoms of the same or different chemical elements.
All bodies consist of a huge number of particles: atoms, molecules and ions. These particles move continuously and chaotically. Their movement does not have any specific direction and is called thermal movement . During their motion, particles interact with each other through absolutely elastic collisions.
We cannot observe molecules and atoms with the naked eye. But we can see the result of their actions.
Confirmation of the main provisions of the molecular kinetic theory are: diffusion , Brownian motion And change aggregate states of substances .
Diffusion
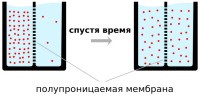
Diffusion in liquid
One of the proofs constant movement molecules - phenomenon diffusion .
In the process of movement, molecules and atoms of one substance penetrate between the molecules and atoms of another substance in contact with it. Molecules and atoms of the second substance behave in exactly the same way. in relation to the first. And after some time, the molecules of both substances are evenly distributed throughout the entire volume.
The process of penetration of molecules of one substance between molecules of another is called diffusion . We encounter the phenomenon of diffusion at home every day when we put a tea bag into a glass of boiling water. We observe how colorless boiling water changes its color. By throwing several manganese crystals into a test tube with water, you can see that the water turns pink. This is also diffusion.
The number of particles per unit volume is called concentration substances. During diffusion, molecules move from those parts of a substance where the concentration is higher to those parts where it is lower. The movement of molecules is called diffusion flow . As a result of the diffusion of concentration into various parts substances are leveled out.
Diffusion can be observed in gases, liquids and solids. In gases it occurs at a faster rate than in liquids. We know how quickly odors spread in the air. The liquid in a test tube turns color much more slowly if ink is dropped into it. And if we put crystals of table salt at the bottom of a container with water and do not mix, then more than one day will pass before the solution becomes homogeneous.
Diffusion also occurs at the boundary of contacting metals. But its speed in this case is very low. If you coat copper with gold, then at room temperature and atmospheric pressure gold will penetrate copper only a few microns in a few thousand years.
Lead from an ingot placed under a weight on a gold ingot will penetrate into it only to a depth of 1 cm in 5 years.
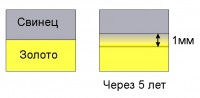
Diffusion in metals
Diffusion rate
The rate of diffusion depends on the cross-sectional area of the flow, the difference in concentrations of substances, the difference in their temperatures or charges. Through a rod with a diameter of 2 cm, heat spreads 4 times faster than through a rod with a diameter of 1 cm. The higher the temperature difference between substances, the higher the rate of diffusion. During thermal diffusion, its speed depends on thermal conductivity material, and in the case of flow electric charges- from electrical conductivity .
Fick's law

Adolf Fick
In 1855, the German physiologist Adolf Eugen Fick made the first quantitative description of diffusion processes:
Where J - density diffusion flow of matter,
D - diffusion coefficient,
C - substance concentration.
Substance diffusion flux densityJ [cm -2 s -1 ] is proportional to the diffusion coefficientD [cm -2 s -1 ] and the concentration gradient taken with the opposite sign.
This equation is called Fick's first equation .
Diffusion, as a result of which the concentrations of substances are equalized, is called non-stationary diffusion . With such diffusion, the concentration gradient changes with time. And in case stationary diffusion this gradient remains constant.
Brownian motion

Robert Brown
This phenomenon was discovered by the Scottish botanist Robert Brown in 1827, studying under a microscope cytoplasmic grains suspended in water, isolated from pollen cells of a North American plant.Clarkia pulchella, he paid attention to the smallest solid grains. They trembled and moved slowly for no apparent reason. If the temperature of the liquid increased, the speed of the particles increased. The same thing happened when the particle size decreased. And if their size increased, the temperature of the liquid decreased or its viscosity increased, the movement of the particles slowed down. And these amazing “dances” of particles could be observed for an infinitely long time. Deciding that the reason for this movement was that the particles were alive, Brown replaced the grains with small particles of coal. The result was the same.

Brownian motion
To repeat Brown's experiments, it is enough to have the most ordinary microscope. The molecular size is too small. And it is impossible to examine them with such a device. But if we tint water in a test tube with watercolor paint and then look at it through a microscope, we will see tiny colored particles moving randomly. These are not molecules, but particles of paint suspended in water. And they are forced to move by water molecules that hit them from all sides.
This is the behavior of all particles visible through a microscope that are suspended in liquids or gases. Their random movement caused by the thermal movement of molecules or atoms is called Brownian motion . A Brownian particle is continuously subjected to impacts from the molecules and atoms that make up liquids and gases. And this movement does not stop.
But Brownian motion can involve particles as small as 5 microns (micrometers). If their size is larger, they are immobile. The smaller the size of a Brownian particle, the faster it moves. Particles smaller than 3 microns move translationally along all complex trajectories or rotate.
Brown himself could not explain the phenomenon he discovered. And only in the 19th century did scientists find the answer to this question: the movement of Brownian particles is caused by the influence of the thermal movement of molecules and atoms on them.
Three states of matter
The molecules and atoms that make up matter are not only in motion, but also interact with each other, mutually attracting or repelling.
If the distance between the molecules is comparable to their size, then they experience attraction. If it becomes smaller, then the repulsive force begins to dominate. This explains the resistance of physical bodies to deformation (compression or tension).
If the body is compressed, the distance between the molecules decreases, and repulsive forces will try to return the molecules to their original state. When stretching, the deformation of the body will interfere with the forces of attraction between the molecules.
Molecules interact not only within one body. Dip a piece of fabric into the liquid. We'll see that it gets wet. This is explained by the fact that liquid molecules are attracted to solid molecules more strongly than to each other.
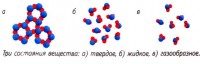
Each physical substance depending on temperatures and pressures, it can be in three states: solid, liquid or gaseous . They're called aggregate .
In gases the distance between molecules is large. Therefore, the forces of attraction between them are so weak that they perform chaotic and almost free movement in space. They change the direction of their movement, hitting each other or the walls of blood vessels.
In liquids molecules are located closer to each other than in a gas. The force of attraction between them is greater. The molecules in them no longer move freely, but oscillate chaotically around the equilibrium position. But they are able to jump in the direction of the action of an external force, changing places with each other. The result of this is fluid flow.
In solids The interaction forces between molecules are very strong due to the close distance between them. They cannot overcome the attraction of neighboring molecules, therefore they are only able to perform oscillatory movements around the equilibrium position.
Solids retain volume and shape. Liquid has no shape; it always takes the shape of the vessel in which it is currently located. But its volume remains the same. Gaseous bodies behave differently. They easily change both shape and volume, taking the shape of the vessel in which they were placed and occupying the entire volume provided to them.
However, there are also bodies that have a liquid structure, have little fluidity, but are still able to maintain their shape. Such bodies are called amorphous .
Modern physics also identifies a fourth state of matter - plasma .
Basic principles of molecular kinetic theory
What is the main task of molecular physics? What is molecular kinetic theory? Formulate the main provisions of the molecular kinetic theory. What observations and experiments confirm the main provisions of the molecular kinetic theory? What is a molecule? atom?
What is the main task of molecular physics? Explain the properties of macroscopic bodies and the thermal processes occurring in them, based on the idea that all bodies consist of individual, randomly moving particles.
What is molecular kinetic theory? Molecular kinetic theory (MKT) is a theory that considers the structure of matter from the point of view of three main approximately correct positions.
Formulate the main provisions of the molecular kinetic theory. all bodies consist of particles with gaps between them; particles are in continuous chaotic motion; particles interact with each other
What observations and experiments confirm the main provisions of the molecular kinetic theory? Diffusion Brownian motion Dunoyer and Stern experiments
What is a molecule? atom? A molecule is an independent particle, an essential component of any substance, which has all chemical and physical properties of this substance. Any molecule consists of the simplest independent particles - atoms. An atom is the smallest possible particle of any of the simplest chemical substances called elements.
The main provisions of the molecular kinetic theory were subjected to comprehensive experimental testing. The most famous experiments demonstrating the molecular structure of matter and confirming the molecular kinetic theory are the Dunoyer experiment and the experiment of Otto Stern (1888 - 1969), performed in 1911 and 1920, respectively. In these experiments, molecular beams were created by evaporating various metals, and therefore the molecules of the gases under study were atoms of these metals. Such experiments made it possible to verify the predictions of the molecular kinetic theory that it makes for the case of gases whose molecules can be considered as material points, that is, for monatomic gases.
Dunoyer's experiment The scheme of Dunoyer's experiment with molecular beams is shown in Fig. 1. A glass vessel, the material of which was chosen to ensure a high vacuum, was divided into three compartments 1, 2 and 3 by two partitions with diaphragms 4. In compartment 1 there was a gas, which in this experiment was used as sodium vapor obtained by heating it. The molecules of this gas could freely fly through the holes in the diaphragms, collimating the molecular beam 5, that is, allowing it to pass only within a small solid angle. In compartments 2 and 3, an ultra-high vacuum was created, such that sodium atoms could fly through them without colliding with air molecules. An unscattered molecular beam left a trace 6 on the end wall of the vessel. But even in the case of ultrahigh vacuum, the molecular beam was scattered at the edges of the diaphragms 4. Therefore, on the end wall of the vessel there was a “penumbra” region 7, in which traces of particles that had undergone scattering were left. As the vacuum in compartment 3 deteriorated, area 7 increased. Based on the blurring of the trace of scattered sodium atoms, it was possible to estimate their mean free path. Such assessments were carried out by Max Born (1882 - 1970) based on the results of experiments similar to Dunoyer's experiment.
Otto Stern's experiment Some of the most famous experiments with molecular beams were Stern's experiments, in which direct measurements of molecular velocities were made for the first time. The most famous scheme of Stern's experiment is shown in Fig. 2. Platinum thread 1, on which a drop of silver was applied, was located on the axis of two coaxial cylinders 2 and 3, and in cylinder 2 there was a slot parallel to its axis. The cylinders could rotate around their axis. In Stern's experiments, the angular speed of their rotation was 2...3 thousand revolutions per minute. When passed through a platinum thread electric current it heated up to a maximum temperature of about 1200 oC. As a result of this, silver began to evaporate, its atoms flew through the slit 4 of cylinder 2 and settled on the surface of cylinder 3, leaving a trace 5 on it. For non-rotating cylinders, silver atoms, moving rectilinearly, more or less uniformly settled on the surface of the outer cylinder, inside sector corresponding to their linear distribution. The rotation of the cylinders led to a curvature of the trajectory of the molecules in the reference frame associated with the cylinders and, as a consequence, to a change in the position of the silver atoms deposited on the outer cylinder. By analyzing the density of settled molecules, it was possible to estimate the characteristics of the distribution of molecules by speed, in particular, the maximum and minimum speeds corresponding to the edges of the trace, and also to find the most probable speed corresponding to the maximum density of settled molecules.
Rice. 1 - Scheme of the Dunoyer experiment 1 - compartment filled with gas 2 and 3 - compartments with ultra-high vacuum 4 - partitions with diaphragms 5 - molecular beam 6 - trace of an unscattered beam 7 - trace of scattered molecules
Fig. 2 - Stern experiment diagram 1 - source of molecules 2 and 3 - rotating cylinders 4 - slit limiting the molecular beam 5 - trace of the molecular beam
Diffusion Diffusion is the phenomenon of mutual penetration of a molecule of one substance between the molecules of another. Diffusion can occur in gases (very fast), in liquids (fast), in metals (very slowly).
Brownian motion Brownian motion is the thermal movement of particles suspended in a liquid or gas. The causes of Brownian motion are: 1. Random movement of molecules 2. Deviation from the average value of the pressure they produce. The following have been established: 1. Brownian motion occurs at any t and lasts indefinitely. 2. Brownian particles make random trajectories. 3. The nature of the motion of a Brownian particle does not depend on its nature. Brownian motion is direct evidence of the random motion of molecules.
