The structure of amino acid radicals is extremely diverse. This circumstance allows the use of numerous color reactions to detect most amino acids. Many of them are so sensitive and highly specific that they make it possible to detect minute amounts of individual amino acids in complex mixtures, biological fluids, protein hydrolysates, etc. Therefore, some color reactions are used to quantify amino acids.
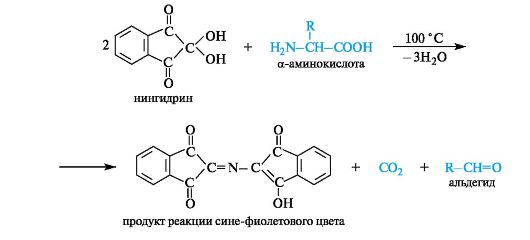
Interaction with ninhydrin is one of the most sensitive reactions to free a-amino groups in amino acids. When heated, proteins, polypeptides and amino acids form blue or blue-violet compounds with ninhydrin, with the exception of proline - the products of its reaction with ninhydrin are bright yellow. The essence of the reaction is that a-amino acids, reacting with ninhydrin, undergo oxidative deamination and decarboxylation at the same time.
One of the reaction products is hydrindanthine reacts with ammonia and another ninhydrin molecule to form a colored compound, diketohydrinylidene-diketohydrindamine (DIDA).
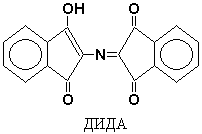
Ninhydrin is capable of reacting not only with a-amino acids, but also with other amines - in this case the same color develops, but without the release of CO 2. Consequently, the release of CO 2 is an indicator of the participation of a-amino acids in the ninhydrin reaction. Peptides and NH 3 also react with ninhydrin, but less actively than a-amino acids.
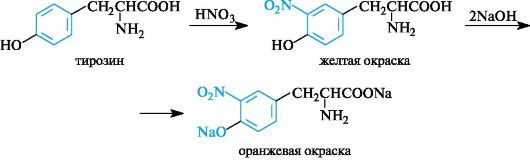
Xanthoprene reaction specific for radicals aromatic amino acids. Its essence is that when aromatic amino acids or proteins and polypeptides containing them are heated with concentrated nitric acid, yellow nitro compounds are formed. The reaction occurs in two stages. In the first stage, the benzene ring of the amino acid undergoes nitration.
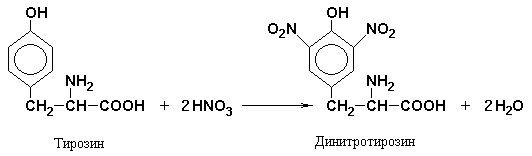
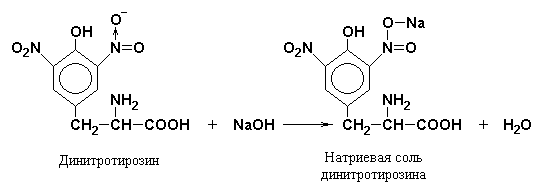
In the second stage, nitration products react with sodium or ammonium hydroxide to form sodium or ammonium salt, which has a yellow-orange color.
When carrying out the xanthoprene reaction, gelatin, which does not contain aromatic amino acids, is used as a control.
Foll reaction specific to sulfur-containing amino acids– cysteine, cystine, methionine or for proteins containing these amino acids. The reaction occurs in two stages. First, under the influence of sodium hydroxide upon heating, the SH groups of amino acids are eliminated and sulfur is transferred from organic compound to inorganic:
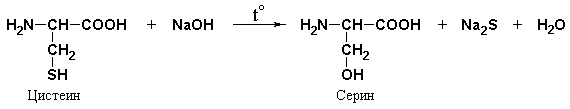
Sodium sulfide then reacts with lead acetate, resulting in the formation of lead sulfide, which precipitates as a black precipitate.
QUALITATIVE REACTIONS TO AMINO ACIDS AND PROTEINS
Goal of the work: study qualitative reactions used to detect proteins and determine their amino acid composition
Color reactions
To detect proteins, color reactions are used, which are divided into two types: general or universal and specific. Universal reactions include biuret (to peptide bonds) and ninhydrate (to α-amino acids). With their help, you can detect any protein. Specific reactions include reactions to individual amino acids, which make it possible to detect specific functional groups in the composition of amino acid radicals. Color reactions to proteins underlie methods for establishing the protein nature of substances, studying the amino acid composition and quantitative content of proteins.
Work 1. Biuret reaction (Piotrovsky reaction).
In an alkaline environment, proteins, as well as their hydrolysis products (polypeptides), give a violet or red-violet color with copper sulfate. The reaction is due to the presence of peptide bonds in the beams, which form colored salt-like complex compounds. The intensity of the color depends on the number of peptide bonds in the molecule and the amount of copper salt.
The reaction gets its name from the urea derivative, biuret, which gives this reaction. Biuret is formed when urea is heated and ammonia is separated from it:
Two molecules of the dienol form of biuret interact with copper (II) hydroxide formed in an alkaline environment. The reaction product is a complex compound (colored copper-sodium salt of biuret), in which coordination bonds are formed due to electron pairs of nitrogen atoms of imine groups:
1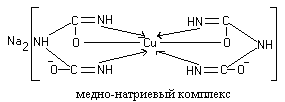
Colored copper-sodium salts of peptides and proteins are constructed in a similar way.
The biuret reaction is also produced by asparagine (aspartic acid amide) and the amino acids histidine, serine, and threonine.
Progress. 10-12 drops of egg or vegetable protein solution are poured into one test tube, 20-30 mg of urea is poured into another and heated on an alcohol lamp until the smell of ammonia disappears and cooled. Add 10 drops of a 10% sodium hydroxide solution and 1-2 drops of a 1% copper (II) sulfate solution to both test tubes. A blue-violet or red-violet color appears in both test tubes.
Work 2. Ninhydrin reaction.
Proteins, polypeptides and amino acids when heated with ninhydrin give a blue and blue-violet color. The ninhydrin reaction is due to the presence of α-amino acids and is one of the most sensitive α-amino groups for detection.
The essence of the reaction is that α-amino acids and peptides, reacting with ninhydrin, undergo oxidative deamination and decarboxylation:
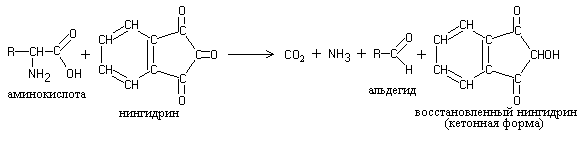
Reduced ninhydrin reacts with ammonia and a second ninhydrin molecule, resulting in the formation of a complex colored compound with a murexide structure:
1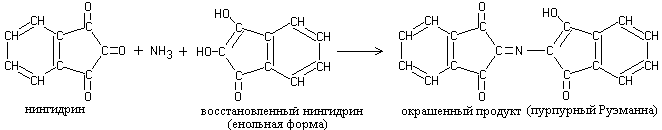
Progress. Pour into two test tubes: into one 10 drops of an egg or vegetable protein solution, into the other 10 drops of a 0.1 percent glycine solution. Add 2-3 drops of a 0.1% ninhydrin solution to each of them and heat. After 1-2 minutes, a pink, then red, and then blue color appears.
Work 3. Xanthoprotein reaction (Mulder reaction)
When solutions of most proteins are heated with concentrated nitric acid, a yellow color is formed, which turns orange in an alkaline solution.
The reaction is due to the presence of cyclic amino acids, which, when interacting with nitric acid, form yellow nitro derivatives, for example:
1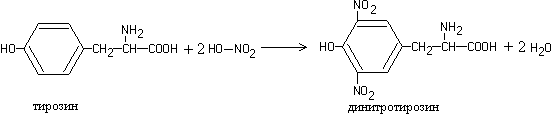
The nitration products of cyclic amino acids react with sodium hydroxide or ammonium hydroxide to form the corresponding orange-colored salts:
1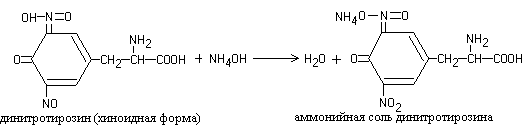
Progress. 8-10 drops of egg or vegetable protein are poured into a test tube, 3-5 drops of concentrated nitric acid are added and heated. A yellow color appears in the test tube. After cooling, an excess of concentrated ammonia solution or 30% sodium hydroxide solution is added to the mixture. The yellow color turns to orange.
Work 4. Reaction to tyrosine (Millon reaction)
Heating most proteins with Millon's reagent (a solution of nitrates and nitrites of mercury (I) and (II) in nitric acid) leads to the formation of a red precipitate.
The reaction is due to the presence of the amino acid tyrosine in the protein, which, when interacted with Millon’s reagent, produces a nitroso derivative, the mercury compound of which is colored red:
1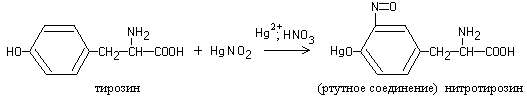
Progress. Add 2-3 drops of Millon's reagent to 8-10 drops of protein solution and carefully heat it. The liquid turns red and a red-brown precipitate forms.
Work 5. Diazoreaction to histidine and tyrosine (Pauli reaction)
When a diazo reagent is added to an alkaline protein solution, the liquid acquires an orange-red color.
The reaction is due to the presence of the amino acids histidine and tyrosine in the protein, which, when reacting with diazobenzenesulfonic acid, form a red azo dye:
1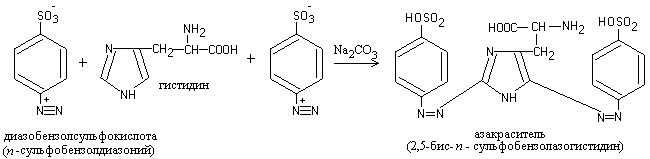
Progress. To the freshly prepared diazo reagent (3 drops of a 1% solution of sulfanilic acid in a 2% solution of hydrochloric acid and 3 drops of a 5% solution of sodium nitrite), add 6-8 drops of a protein solution and, after mixing, 3-5 drops of a 10% solution of sodium carbonate. An intense red color develops.
Work 6. Reactions to tryptophan
The reactions are based on the ability of tryptophan in an acidic environment to interact with aldehydes, thereby forming colored condensation products.
a) Adamkiewicz reaction. The reaction of tryptophan with glyoxylic acid (which is always present in glacial acetic acid) leads to the formation of a red-violet compound:
1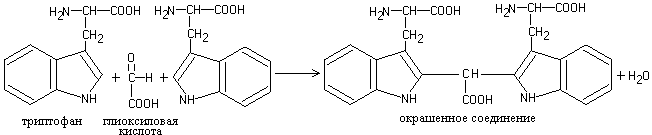
Progress. Add 5 drops of concentrated acetic acid to 5-6 drops of protein solution, heat slightly and layer (carefully!!! along the wall of an inclined test tube) an equal volume of concentrated sulfuric acid. A red-violet ring appears at the boundary of the two layers of liquid.
b) Schultz-Raspail reaction. Tryptophan, interacting with hydroxymethylfurfural (formed during the hydrolysis of sucrose and dehydration of monosaccharides under the action of concentrated sulfuric acid) forms a cherry-red complex.
Progress. Add 1 drop of 10% sucrose solution to 5-6 drops of protein solution and add 1 ml of concentrated sulfuric acid. A cherry-red color appears at the interface between liquids.
Work 7. Foll reaction to sulfur-containing amino acids
Heating protein with alkali and plumbite leads to the appearance of a brown or black precipitate. The reaction is due to the presence of sulfur-containing amino acids in the protein, which are destroyed by alkali to form alkali metal sulfide; the latter with plumbite gives a precipitate of lead sulfide:
1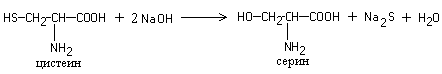
Pb(CH 3 COO) 2 + 4 NaOH → Na 2
Na 2 + Na 2 S → PbS↓ + 4 NaOH
Progress. To 5-6 drops of protein solution add 10 drops of a 30% sodium hydroxide solution and 1 drop of a 5% lead acetate solution. When heated for a long time, a black precipitate of lead sulfide precipitates.
Control definition
All studied reactions to amino acids and proteins are carried out with a control protein solution, on the basis of which a conclusion is made about the amino acid composition of the protein. The results of the experiments are entered into the table.
The report is drawn up indicating the purpose of the work, includes reaction equations, experimental data and conclusions. The findings contain data on the amino acid composition of proteins and the possibility of their detection by color reactions.
Ninhydrin reaction, a color reaction used for the qualitative and quantitative determination of amino acids, imino acids and amines. When heated in an alkaline environment, ninhydrin (tricetohydrin dehydrate, C 9 H b O 4) with substances having primary amino groups (-NH 2), a product is formed that has a stable intense blue-violet color with a maximum absorption of about 570 nm. Since absorption at this wavelength depends linearly on the number of free amino groups, the ninhydrin reaction served as the basis for their quantitative determination by colorimetry or spectrophotometry. This reaction is also used to determine secondary amino groups (>NH) in imino acids - proline and hydroxyproline; in this case, a bright yellow product is formed. Sensitivity - up to 0.01%. Modern automatic amino acid analysis is carried out by combining ion exchange separation of amino acids and their quantitative determination using the ninhydrin reaction. When separating mixtures of amino acids using paper chromatography, it makes it possible to determine each amino acid in an amount of at least 2-5 μg.
The intensity of the color can be used to judge the amount of amino acids.
This reaction is positive not only with free amino acids, but also with peptides, proteins, etc.
Xanthoprotein reaction allows the detection of aromatic amino acids (phenylalanine, tyrosine, histidine, tryptophan), based on the reaction of electrophilic substitution in the aromatic nucleus (nitration).
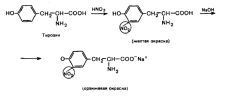
When concentrated nitric acid acts, for example, on tyrosine, a yellow-colored product is formed.
Biuret reaction- used as a color reaction to proteins. In an alkaline environment in the presence of copper(II) salts, they give a violet color. The color is due to the formation of a copper(II) complex compound due to the peptide group -CO-NH-, which is characteristic of proteins. This reaction got its name from a urea derivative - biuret, which is formed when urea is heated with the elimination of ammonia:
In addition to proteins and biuret, the same coloring is given by other compounds containing this group: amides, imides of carboxylic acids, as well as compounds containing the groups -CS-NH- or =CH-NH- in the molecule. Proteins, some amino acids, peptides, biuret and medium peptones also react.
The color of the complex obtained by the biuret reaction with various peptides is somewhat different and depends on the length of the peptide chain. Peptides with a chain length of four amino acid residues and above form a red complex, tripeptides - purple, and dipeptides - blue.
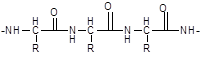
ketone form of the polypeptide
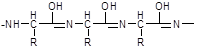
enol form of the polypeptide
Education peptide bond.
Intermolecular interaction of α-amino acids leads to the formation of peptides. When two α-amino acids interact, a dipeptide is formed.
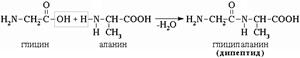
The intermolecular interaction of three α-amino acids leads to the formation of a tripeptide, etc.
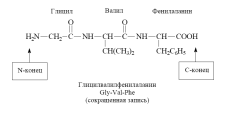
Fragments of amino acid molecules that form a peptide chain are called amino acid residues, and the CO–NH bond is called peptide bond.
22. Decarboxylation of α-amino acids - formation biogenic amines and bioregulators (histamine, tryptamine).
Some amino acids and their derivatives can undergo decarboxylation - the removal of the oc-carboxyl group. In mammalian tissues, a number of amino acids or their derivatives can undergo decarboxylation: Tri, Tyr, Val, Gis, Glu, Cis, Apr, etc. The reaction products are CO 2 and amines, which have a pronounced biological effect on the body (biogenic amines):
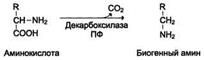
Decarboxylation reactions are irreversible and are catalyzed by decarboxylase enzymes. The prosthetic group of decarboxylases in animal cells is pyridoxal phosphate.
Amines formed by decarboxylation of amino acids are often biologically active substances. They function as neurotransmitters (serotonin, dopamine, GABA, etc.), hormones (norepinephrine, adrenaline), and local regulatory factors (histamine, carnosine, spermine, etc.).
Histamine is formed by decarboxylation of the amino acid histidine. It is synthesized in mast cells, accumulates in secretory granules, and is released when cells are irritated.
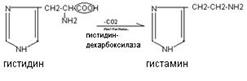
Histamine has a variety of biological effects: it causes vasodilation, reduces blood pressure, increases tissue permeability, causes local edema, stimulates gastric secretion, and has a bronchospatic effect. In high concentrations, it is a mediator of inflammatory and allergic reactions.
Serotonin is formed by decarboxylation of hydroxytryptophan. It is synthesized in chromaffin cells, in some nuclei of subcortical structures, and platelets.
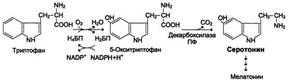
Effects of serotonin: causes vasospasm, increased blood pressure, stimulates intestinal motility, participates in thermoregulation, sleep mechanisms, is a source for the synthesis of the hormone melatonin, affects human mental reactions. Thus, in schizophrenia there is a disturbance in serotonin metabolism.
Catecholamines (dopamine, adrenaline, norepinephrine) are synthesized from the amino acid tyrosine.
Dopamine is an excitatory neurotransmitter; when it is deficient, Parkinson's disease develops (adynamia, rigidity, tremor). Adrenaline causes vasospasm, increases blood pressure, stimulates the heart, and is a hormone.
Norepinephrine primarily performs neurotransmitter functions.
Polyamines (spermine, spermidine) are synthesized from ornithine and methionine, are part of chromatin, and are involved in the regulation of translation, transcription, and replication processes.
Since biogenic amines are very active, they are quickly inactivated in tissues. Decomposition of biogenic amines carried out in several ways: oxidation, methylation, deamination. The main method of inactivation of biogenic amines is oxidative deamination under the influence of amine oxidases enzymes (monoamine oxidases, polyamine oxidases).
![]()
Amino acids can be covalently linked to each other using peptide bonds. The carboxyl group of one amino acid is covalently bonded to the amino group of another amino acid. This creates an R-CO-NH-R bond, called a peptide bond. In this case, the water molecule is split off.
Proteins form the material basis of the chemical activity of the cell. The functions of proteins in nature are universal. Name proteins, the most accepted term in Russian literature corresponds to the term proteins(from Greek proteios- first). To date, great strides have been made in establishing the relationship between the structure and functions of proteins, the mechanism of their participation in the most important processes of the body's life, and in understanding the molecular basis of the pathogenesis of many diseases.
Depending on their molecular weight, peptides and proteins are distinguished. Peptides have a lower molecular weight than proteins. Peptides are more likely to have a regulatory function (hormones, enzyme inhibitors and activators, ion transporters across membranes, antibiotics, toxins, etc.).
12.1. α -Amino acids
12.1.1. Classification
Peptides and proteins are built from α-amino acid residues. Total number There are more than 100 naturally occurring amino acids, but some of them are found only in a certain community of organisms; the 20 most important α-amino acids are constantly found in all proteins (Scheme 12.1).
α-Amino acids are heterofunctional compounds whose molecules contain both an amino group and a carboxyl group at the same carbon atom.
Scheme 12.1.The most important α-amino acids*
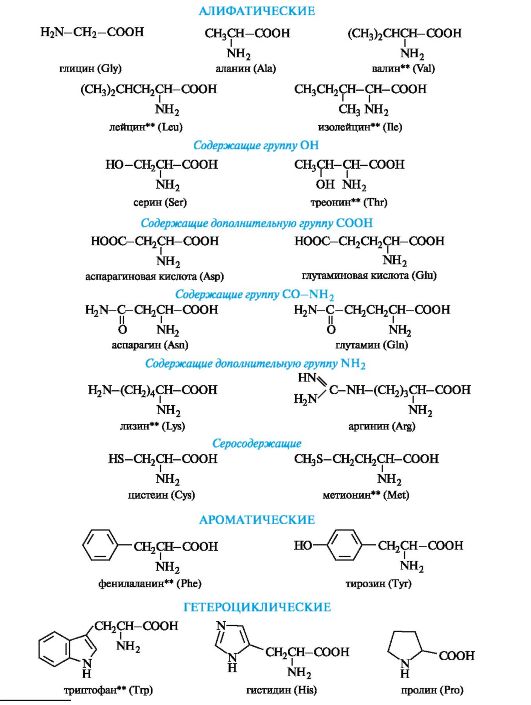
* Abbreviations are used only to write amino acid residues in peptide and protein molecules. ** Essential amino acids.
The names of α-amino acids can be constructed using substitutive nomenclature, but their trivial names are more often used.

Trivial names for α-amino acids are usually associated with sources of isolation. Serine is part of silk fibroin (from lat. serieus- silky); Tyrosine was first isolated from cheese (from the Greek. tyros- cheese); glutamine - from cereal gluten (from German. Gluten- glue); aspartic acid - from asparagus sprouts (from lat. asparagus- asparagus).
Many α-amino acids are synthesized in the body. Some amino acids necessary for protein synthesis are not produced in the body and must come from outside. These amino acids are called irreplaceable(see diagram 12.1).
Essential α-amino acids include:
valine isoleucine methionine tryptophan
leucine lysine threonine phenylalanine
α-Amino acids are classified in several ways depending on the characteristic that serves as the basis for their division into groups.
One of the classification features is chemical nature radical R. Based on this feature, amino acids are divided into aliphatic, aromatic and heterocyclic (see diagram 12.1).
Aliphaticα -amino acids. This is the largest group. Within it, amino acids are divided using additional classification features.
Depending on the number of carboxyl groups and amino groups in the molecule, the following are distinguished:
Neutral amino acids - one NH group each 2 and COOH;
Basic amino acids - two NH groups 2 and one group
COOH;
Acidic amino acids - one NH 2 group and two COOH groups.
It can be noted that in the group of aliphatic neutral amino acids the number of carbon atoms in the chain does not exceed six. At the same time, there are no amino acids with four carbon atoms in the chain, and amino acids with five and six carbon atoms have only a branched structure (valine, leucine, isoleucine).
An aliphatic radical may contain “additional” functional groups:
Hydroxyl - serine, threonine;
Carboxylic - aspartic and glutamic acids;
Thiol - cysteine;
Amide - asparagine, glutamine.
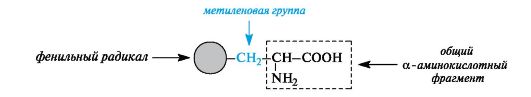
Aromaticα -amino acids. This group includes phenylalanine and tyrosine, constructed in such a way that the benzene rings in them are separated from the common α-amino acid fragment by the methylene group -CH 2-.
Heterocyclic α -amino acids. Histidine and tryptophan belonging to this group contain heterocycles - imidazole and indole, respectively. The structure and properties of these heterocycles are discussed below (see 13.3.1; 13.3.2). The general principle of constructing heterocyclic amino acids is the same as aromatic ones.
Heterocyclic and aromatic α-amino acids can be considered as β-substituted derivatives of alanine.
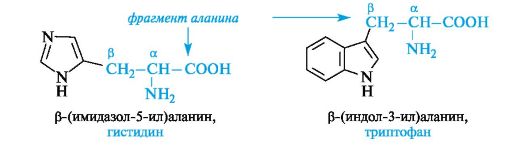
The amino acid also belongs to gerocyclic proline, in which the secondary amino group is included in the pyrrolidine
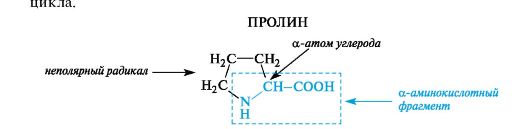
In the chemistry of α-amino acids, much attention is paid to the structure and properties of the “side” radicals R, which play an important role in the formation of the structure of proteins and their performance biological functions. Of great importance are such characteristics as the polarity of the “side” radicals, the presence of functional groups in the radicals and the ability of these functional groups to ionize.
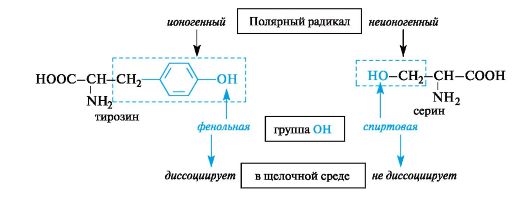
Depending on the side radical, amino acids with non-polar(hydrophobic) radicals and amino acids c polar(hydrophilic) radicals.
The first group includes amino acids with aliphatic side radicals - alanine, valine, leucine, isoleucine, methionine - and aromatic side radicals - phenylalanine, tryptophan.
The second group includes amino acids that have polar functional groups in their radicals that are capable of ionization (ionogenic) or are unable to transform into an ionic state (nonionic) under body conditions. For example, in tyrosine the hydroxyl group is ionic (phenolic in nature), in serine it is nonionic (alcoholic in nature).
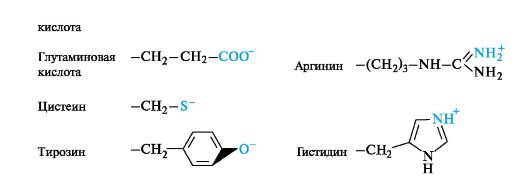
Polar amino acids with ionic groups in radicals under certain conditions can be in an ionic (anionic or cationic) state.
12.1.2. Stereoisomerism
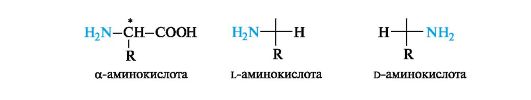
The main type of construction of α-amino acids, i.e., the bond of the same carbon atom with two different functional groups, a radical and a hydrogen atom, in itself predetermines the chirality of the α-carbon atom. The exception is the simplest amino acid glycine H 2 NCH 2 COOH, which has no center of chirality.
The configuration of α-amino acids is determined by the configuration standard - glyceraldehyde. The location of the amino group in the standard Fischer projection formula on the left (similar to the OH group in l-glyceraldehyde) corresponds to the l-configuration, and on the right - to the d-configuration of the chiral carbon atom. By R, In the S-system, the α-carbon atom in all α-amino acids of the l-series has an S-configuration, and in the d-series, an R-configuration (the exception is cysteine, see 7.1.2).
Most α-amino acids contain one asymmetric carbon atom per molecule and exist as two optically active enantiomers and one optically inactive racemate. Almost all natural α-amino acids belong to the l-series.
The amino acids isoleucine, threonine and 4-hydroxyproline contain two chirality centers in the molecule.
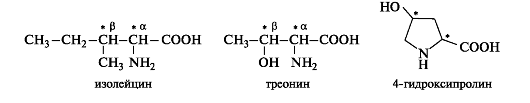
Such amino acids can exist as four stereoisomers, representing two pairs of enantiomers, each of which forms a racemate. To build animal proteins, only one of the enantiomers is used.
The stereoisomerism of isoleucine is similar to the previously discussed stereoisomerism of threonine (see 7.1.3). Of the four stereoisomers, proteins contain l-isoleucine with the S configuration of both asymmetric carbon atoms C-α and C-β. The names of another pair of enantiomers that are diastereomers with respect to leucine use the prefix Hello-.
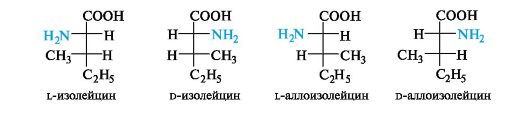
Cleavage of racemates. The source of α-amino acids of the l-series are proteins, which are subjected to hydrolytic cleavage for this purpose. Due to the great need for individual enantiomers (for the synthesis of proteins, medicinal substances, etc.) chemical methods for breaking down synthetic racemic amino acids. Preferred enzymatic method of digestion using enzymes. Currently, chromatography on chiral sorbents is used to separate racemic mixtures.
12.1.3. Acid-base properties
The amphotericity of amino acids is determined by acidic (COOH) and basic (NH 2) functional groups in their molecules. Amino acids form salts with both alkalis and acids.
In the crystalline state, α-amino acids exist as dipolar ions H3N+ - CHR-COO- (commonly used notation
The structure of the amino acid in non-ionized form is for convenience only).
IN aqueous solution amino acids exist as an equilibrium mixture of dipolar ion, cationic and anionic forms.
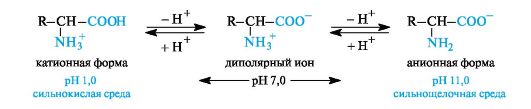
The equilibrium position depends on the pH of the medium. For all amino acids, cationic forms predominate in strongly acidic (pH 1-2) and anionic forms in strongly alkaline (pH > 11) environments.
The ionic structure determines a number of specific properties of amino acids: high melting point (above 200? C), solubility in water and insolubility in non-polar organic solvents. The ability of most amino acids to dissolve well in water is an important factor in ensuring their biological functioning; the absorption of amino acids, their transport in the body, etc. are associated with it.
A fully protonated amino acid (cationic form), from the standpoint of Brønsted’s theory, is a dibasic acid,
By donating one proton, such a dibasic acid turns into a weak monobasic acid - a dipolar ion with one acid group NH 3 + . Deprotonation of the dipolar ion leads to the production of the anionic form of the amino acid - the carboxylate ion, which is a Brønsted base. The values characterize
The basic acidic properties of the carboxyl group of amino acids usually range from 1 to 3; values pK a2 characterizing the acidity of the ammonium group - from 9 to 10 (Table 12.1).
Table 12.1.Acid-base properties of the most important α-amino acids
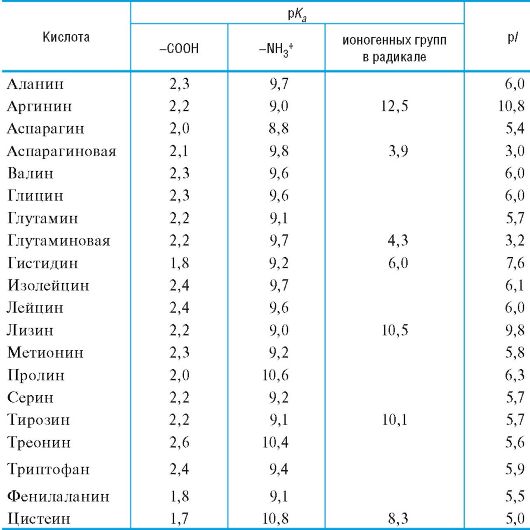
Equilibrium position, i.e. ratio various forms amino acids in an aqueous solution at certain pH values significantly depends on the structure of the radical, mainly on the presence of ionic groups in it, which play the role of additional acidic and basic centers.
The pH value at which the concentration of dipolar ions is maximum, and the minimum concentrations of cationic and anionic forms of an amino acid are equal, is calledisoelectric point (p/).
Neutralα -amino acids. These amino acids matterpIslightly lower than 7 (5.5-6.3) due to the greater ability to ionize the carboxyl group under the influence of the -/- effect of the NH 2 group. For example, alanine has an isoelectric point at pH 6.0.
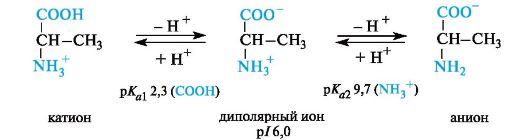
Sourα -amino acids. These amino acids have an additional carboxyl group in the radical and are in a fully protonated form in a strongly acidic environment. Acidic amino acids are tribasic (according to Brøndsted) with three meaningspK a,as can be seen in the example of aspartic acid (p/ 3.0).
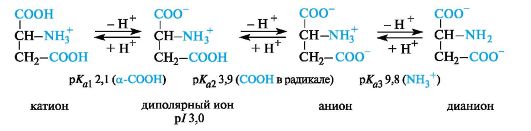
For acidic amino acids (aspartic and glutamic), the isoelectric point is at a pH much lower than 7 (see Table 12.1). In the body at physiological pH values (for example, blood pH 7.3-7.5), these acids are in anionic form, since both carboxyl groups are ionized.
Basicα -amino acids. In the case of basic amino acids, the isoelectric points are located in the pH region above 7. In a strongly acidic environment, these compounds are also tribasic acids, the ionization stages of which are illustrated by the example of lysine (p/ 9.8).
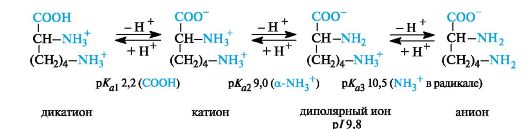
In the body, basic amino acids are found in the form of cations, that is, both amino groups are protonated.
In general, no α-amino acid in vivois not at its isoelectric point and does not fall into a state corresponding to the lowest solubility in water. All amino acids in the body are in ionic form.
12.1.4. Analytically important reactions α -amino acids
α-Amino acids, as heterofunctional compounds, enter into reactions characteristic of both the carboxyl and amino groups. Some chemical properties of amino acids are due to the functional groups in the radical. This section discusses reactions that are of practical importance for the identification and analysis of amino acids.
Esterification.When amino acids react with alcohols in the presence of an acid catalyst (for example, hydrogen chloride gas), they produce esters in the form of hydrochlorides. To isolate free esters, the reaction mixture is treated with ammonia gas.
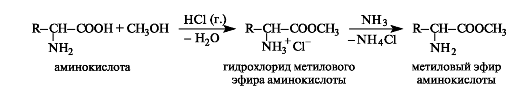
Amino acid esters do not have a dipolar structure, therefore, unlike the parent acids, they dissolve in organic solvents and are volatile. Thus, glycine is a crystalline substance with a high melting point (292°C), and its methyl ester is a liquid with a boiling point of 130°C. Analysis of amino acid esters can be carried out using gas-liquid chromatography.
Reaction with formaldehyde. Of practical importance is the reaction with formaldehyde, which underlies the quantitative determination of amino acids by the method formol titration(Sørensen method).
The amphoteric nature of amino acids does not allow direct titration with alkali for analytical purposes. The interaction of amino acids with formaldehyde produces relatively stable amino alcohols (see 5.3) - N-hydroxymethyl derivatives, the free carboxyl group of which is then titrated with alkali.
Qualitative reactions. A feature of the chemistry of amino acids and proteins is the use of numerous qualitative (color) reactions, which previously formed the basis of chemical analysis. Nowadays, when research is carried out using physicochemical methods, many qualitative reactions continue to be used for the detection of α-amino acids, for example, in chromatographic analysis.
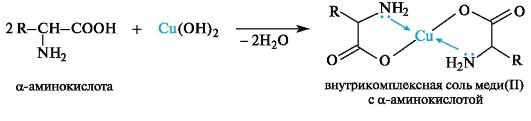
Chelation. With cations of heavy metals, α-amino acids as bifunctional compounds form intra-complex salts, for example, with freshly prepared copper(11) hydroxide under mild conditions, well-crystallizing chelates are obtained
copper salts(11) of blue color(one of the nonspecific methods for detecting α-amino acids).
Ninhydrin reaction. The general qualitative reaction of α-amino acids is the reaction with ninhydrin. The reaction product has a blue-violet color, which is used for visual detection of amino acids on chromatograms (on paper, in a thin layer), as well as for spectrophotometric determination on amino acid analyzers (the product absorbs light in the region of 550-570 nm).
Deamination. In laboratory conditions, this reaction is carried out by the action of nitrous acid on α-amino acids (see 4.3). In this case, the corresponding α-hydroxy acid is formed and nitrogen gas is released, the volume of which is used to determine the amount of amino acid that has reacted (Van-Slyke method).
Xanthoprotein reaction. This reaction is used to detect aromatic and heterocyclic amino acids - phenylalanine, tyrosine, histidine, tryptophan. For example, when concentrated nitric acid acts on tyrosine, a nitro derivative is formed, colored yellow. In an alkaline environment, the color becomes orange due to ionization of the phenolic hydroxyl group and an increase in the contribution of the anion to conjugation.
There are also a number of private reactions that allow the detection of individual amino acids.
Tryptophan detected by reaction with p-(dimethylamino)benzaldehyde in sulfuric acid by the appearance of a red-violet color (Ehrlich reaction). This reaction is used for the quantitative analysis of tryptophan in protein breakdown products.
Cysteine detected through several qualitative reactions based on the reactivity of the mercapto group it contains. For example, when a protein solution with lead acetate (CH3COO)2Pb is heated in an alkaline medium, a black precipitate of lead sulfide PbS is formed, which indicates the presence of cysteine in proteins.
12.1.5. Biologically important chemical reactions
In the body, under the influence of various enzymes, a number of important chemical transformations of amino acids are carried out. Such transformations include transamination, decarboxylation, elimination, aldol cleavage, oxidative deamination, and oxidation of thiol groups.
Transamination is the main pathway for the biosynthesis of α-amino acids from α-oxoacids. The donor of the amino group is an amino acid present in cells in sufficient quantity or excess, and its acceptor is an α-oxoacid. In this case, the amino acid is converted into an oxoacid, and the oxoacid into an amino acid with the corresponding structure of radicals. As a result, transamination is a reversible process of interchange of amino and oxo groups. An example of such a reaction is the production of l-glutamic acid from 2-oxoglutaric acid. The donor amino acid can be, for example, l-aspartic acid.
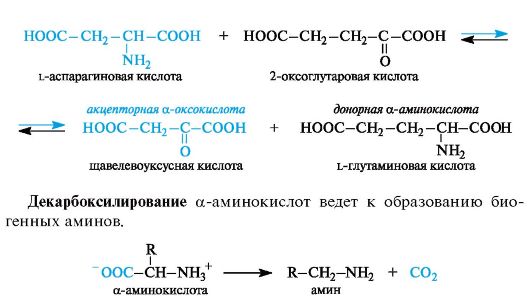
α-Amino acids contain an electron-withdrawing amino group (more precisely, a protonated amino group NH) in the α-position to the carboxyl group 3 +), and therefore capable of decarboxylation.
Eliminationcharacteristic of amino acids in which the side radical in the β-position to the carboxyl group contains an electron-withdrawing functional group, for example, hydroxyl or thiol. Their elimination leads to intermediate reactive α-enamino acids, which easily transform into tautomeric imino acids (analogy with keto-enol tautomerism). As a result of hydration at the C=N bond and subsequent elimination of the ammonia molecule, α-imino acids are converted into α-oxo acids.
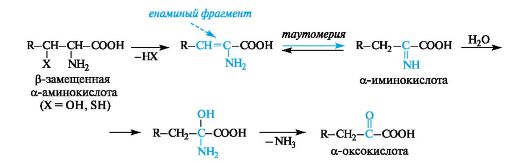
This type of transformation is called elimination-hydration. An example is the production of pyruvic acid from serine.
Aldol cleavage occurs in the case of α-amino acids, which contain a hydroxyl group in the β-position. For example, serine is broken down to form glycine and formaldehyde (the latter is not released in free form, but immediately binds to the coenzyme).
Oxidative deamination can be carried out with the participation of enzymes and the coenzyme NAD+ or NADP+ (see 14.3). α-Amino acids can be converted into α-oxoacids not only through transamination, but also through oxidative deamination. For example, α-oxoglutaric acid is formed from l-glutamic acid. At the first stage of the reaction, glutamic acid is dehydrogenated (oxidized) to α-iminoglutaric acid
acids. In the second stage, hydrolysis occurs, resulting in α-oxoglutaric acid and ammonia. The hydrolysis stage occurs without the participation of an enzyme.
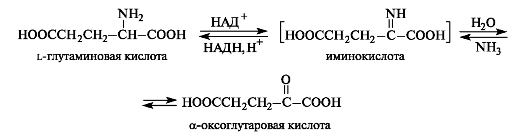
The reaction of reductive amination of α-oxo acids occurs in the opposite direction. α-oxoglutaric acid, always contained in cells (as a product of carbohydrate metabolism), is converted in this way into L-glutamic acid.
Oxidation of thiol groups underlies the interconversions of cysteine and cystine residues, providing a number of redox processes in the cell. Cysteine, like all thiols (see 4.1.2), is easily oxidized to form a disulfide, cystine. The disulfide bond in cystine is easily reduced to form cysteine.
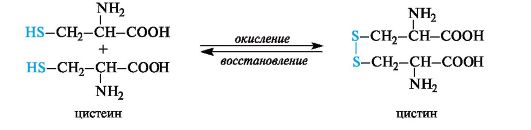
Due to the ability of the thiol group to easily oxidize, cysteine performs a protective function when the body is exposed to substances with high oxidative capacity. In addition, it was the first drug to show anti-radiation effects. Cysteine is used in pharmaceutical practice as a stabilizer for drugs.
Conversion of cysteine to cystine results in the formation of disulfide bonds, such as in reduced glutathione
(see 12.2.3).
12.2. Primary structure of peptides and proteins
Conventionally, it is believed that peptides contain up to 100 amino acid residues in a molecule (which corresponds to a molecular weight of up to 10 thousand), and proteins contain more than 100 amino acid residues ( molecular mass from 10 thousand to several million).
In turn, in the group of peptides it is customary to distinguish oligopeptides(low molecular weight peptides) containing no more than 10 amino acid residues in the chain, and polypeptides, the chain of which includes up to 100 amino acid residues. Macromolecules with a number of amino acid residues approaching or slightly exceeding 100 do not distinguish between polypeptides and proteins; these terms are often used as synonyms.
A peptide and protein molecule can be formally represented as a product of polycondensation of α-amino acids, which occurs with the formation of a peptide (amide) bond between monomer units (Scheme 12.2).
The design of the polyamide chain is the same for the entire variety of peptides and proteins. This chain has an unbranched structure and consists of alternating peptide (amide) groups -CO-NH- and fragments -CH(R)-.
One end of the chain containing an amino acid with a free NH group 2, is called the N-terminus, the other is called the C-terminus,
Scheme 12.2.The principle of constructing a peptide chain
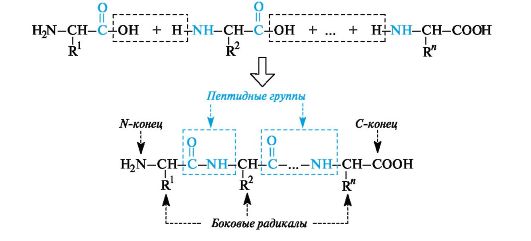
which contains an amino acid with a free COOH group. Peptide and protein chains are written from the N-terminus.
12.2.1. Structure of the peptide group
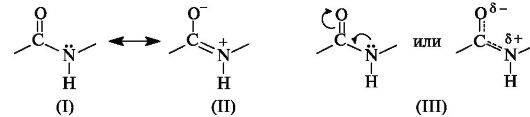
In the peptide (amide) group -CO-NH- the carbon atom is in a state of sp2 hybridization. The lone pair of electrons of the nitrogen atom enters into conjugation with the π-electrons of the C=O double bond. From the standpoint of electronic structure, the peptide group is a three-center p,π-conjugated system (see 2.3.1), the electron density in which is shifted towards the more electronegative oxygen atom. The C, O, and N atoms forming a conjugated system are located in the same plane. The electron density distribution in the amide group can be represented using the boundary structures (I) and (II) or the electron density shift as a result of the +M- and -M-effects of the NH and C=O groups, respectively (III).
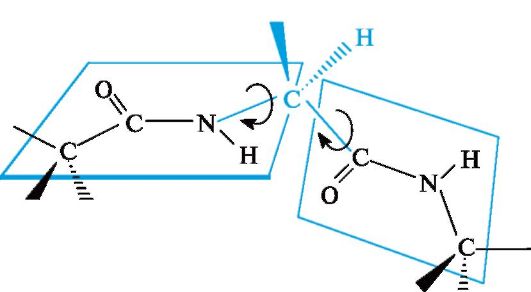
As a result of conjugation, some alignment of bond lengths occurs. The C=O double bond is extended to 0.124 nm compared to the usual length of 0.121 nm, and the C-N bond becomes shorter - 0.132 nm compared to 0.147 nm in the usual case (Fig. 12.1). The planar conjugated system in the peptide group causes difficulty in rotation around the C-N bond (the rotation barrier is 63-84 kJ/mol). Thus, the electronic structure determines a fairly rigid flat structure of the peptide group.
As can be seen from Fig. 12.1, the α-carbon atoms of amino acid residues are located in the plane of the peptide group on opposite sides of the C-N bond, i.e., in a more favorable trans position: the side radicals R of amino acid residues in this case will be the most distant from each other in space.
The polypeptide chain has a surprisingly uniform structure and can be represented as a series of each other located at an angle.
![]()
Rice. 12.1.Planar arrangement of the peptide group -CO-NH- and α-carbon atoms of amino acid residues
to each other planes of peptide groups connected to each other through α-carbon atoms by Cα-N and Cα-Csp bonds 2 (Fig. 12.2). Rotation around these single bonds is very limited due to difficulties in the spatial placement of side radicals of amino acid residues. Thus, the electronic and spatial structure of the peptide group largely determines the structure of the polypeptide chain as a whole.
Rice. 12.2.The relative position of the planes of peptide groups in the polypeptide chain
12.2.2. Composition and amino acid sequence
With a uniformly constructed polyamide chain, the specificity of peptides and proteins is determined by two most important characteristics - amino acid composition and amino acid sequence.
The amino acid composition of peptides and proteins is the nature and quantitative ratio of their α-amino acids.
The amino acid composition is determined by analyzing peptide and protein hydrolysates, mainly by chromatographic methods. Currently, such analysis is carried out using amino acid analyzers.
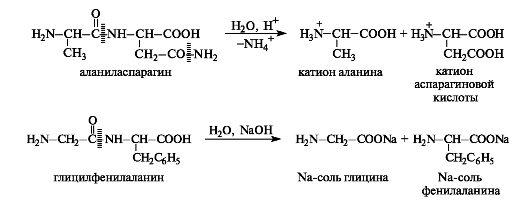
Amide bonds are capable of hydrolysis in both acidic and alkaline environments (see 8.3.3). Peptides and proteins are hydrolyzed to form either shorter chains - this is the so-called partial hydrolysis, or a mixture of amino acids (in ionic form) - complete hydrolysis. Hydrolysis is usually carried out in an acidic environment, since many amino acids are unstable under alkaline hydrolysis conditions. It should be noted that the amide groups of asparagine and glutamine are also subject to hydrolysis.
The primary structure of peptides and proteins is the amino acid sequence, i.e. the order of alternation of α-amino acid residues.
The primary structure is determined by sequentially removing amino acids from either end of the chain and identifying them.
12.2.3. Structure and nomenclature of peptides
Peptide names are constructed by sequentially listing amino acid residues, starting from the N-terminus, with the addition of a suffix-il, except for the last C-terminal amino acid, for which its full name is retained. In other words, the names
amino acids that entered into the formation of a peptide bond due to “their” COOH group end in the name of the peptide with -il: alanil, valyl, etc. (for aspartic and glutamic acid residues the names “aspartyl” and “glutamyl” are used, respectively). The names and symbols of amino acids indicate their belonging to l -row, unless otherwise indicated ( d or dl).
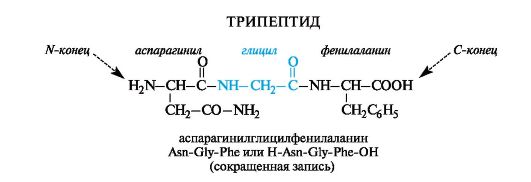
Sometimes in the abbreviated notation the symbols H (as part of an amino group) and OH (as part of a carboxyl group) indicate the unsubstitution of the functional groups of terminal amino acids. This method is convenient for depicting functional derivatives of peptides; for example, the amide of the above peptide at the C-terminal amino acid is written H-Asn-Gly-Phe-NH2.
Peptides are found in all organisms. Unlike proteins, they have a more heterogeneous amino acid composition, in particular, they quite often include amino acids d -row. Structurally, they are also more diverse: they contain cyclic fragments, branched chains, etc.
One of the most common representatives of tripeptides is glutathione- found in the body of all animals, plants and bacteria.
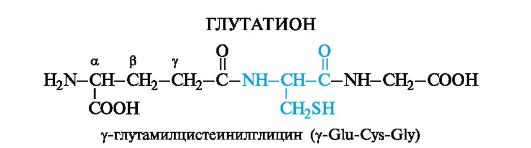
Cysteine in the composition of glutathione makes it possible for glutathione to exist in both reduced and oxidized forms.
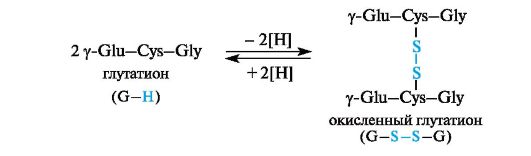
Glutathione is involved in a number of redox processes. It functions as a protein protector, i.e., a substance that protects proteins with free SH thiol groups from oxidation with the formation of disulfide bonds -S-S-. This applies to those proteins for which such a process is undesirable. In these cases, glutathione takes on the action of an oxidizing agent and thus “protects” the protein. During the oxidation of glutathione, intermolecular cross-linking of two tripeptide fragments occurs due to a disulfide bond. The process is reversible.
12.3. Secondary structure of polypeptides and proteins
For high molecular weight polypeptides and proteins along with primary structure characteristic and more high levels organizations that are called secondary, tertiary And quaternary structures.
The secondary structure is described by the spatial orientation of the main polypeptide chain, the tertiary structure by the three-dimensional architecture of the entire protein molecule. Both secondary and tertiary structure are associated with the ordered arrangement of the macromolecular chain in space. The tertiary and quaternary structure of proteins is discussed in a biochemistry course.
It was shown by calculation that one of the most favorable conformations for a polypeptide chain is an arrangement in space in the form of a right-handed helix, called α-helix(Fig. 12.3, a).
The spatial arrangement of an α-helical polypeptide chain can be imagined by imagining that it wraps around a certain
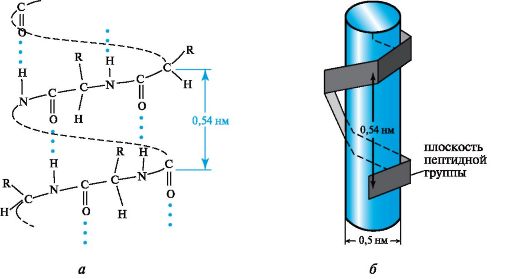
Rice. 12.3.α-helical conformation of the polypeptide chain
cylinder (see Fig. 12.3, b). On average, there are 3.6 amino acid residues per turn of the helix, the pitch of the helix is 0.54 nm, and the diameter is 0.5 nm. The planes of two neighboring peptide groups are located at an angle of 108°, and the side radicals of amino acids are located on the outside of the helix, i.e., they are directed as if from the surface of the cylinder.
The main role in securing such a chain conformation is played by hydrogen bonds, which in the α-helix are formed between the carbonyl oxygen atom of each first and the hydrogen atom of the NH group of each fifth amino acid residue.
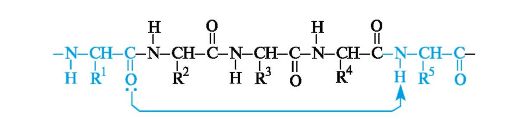
Hydrogen bonds are directed almost parallel to the axis of the α-helix. They keep the chain twisted.
Typically, protein chains are not completely helical, but only partially. Proteins such as myoglobin and hemoglobin contain fairly long α-helical regions, such as the myoglobin chain
75% spiralized. In many other proteins, the proportion of helical regions in the chain may be small.
Another view secondary structure polypeptides and proteins are β-structure, also called folded sheet, or folded layer. Elongated polypeptide chains are arranged in folded sheets, linked by many hydrogen bonds between the peptide groups of these chains (Fig. 12.4). Many proteins contain both α-helical and β-sheet structures.
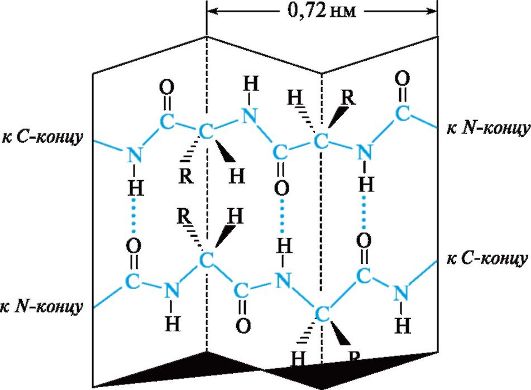
Rice. 12.4.Secondary structure of the polypeptide chain in the form of a folded sheet (β-structure)
