Adiabatic process
Adiabatic is a process in which there is no heat exchange ( = 0) between the physical system and environment. All fast processes are close to adiabatic. For example, the process of sound propagation in a medium can be considered an adiabatic process, since the propagation speed sound wave so great that the exchange of energy between the wave and the medium does not have time to occur. Adiabatic processes are used in internal combustion engines (expansion and compression of the combustible mixture in cylinders), in refrigeration units, etc.
Means for delivering said liquid desiccant gravitationally downwards as free time falling onto said flat surfaces of said flat ribs. The hybrid air conditioning system according to claim 1, wherein said circulation means comprises:
Pumping means for delivering desiccant diluted with water to said condenser, for delivering desorbed concentrated liquid desiccant to the evaporator, and for circulating the desiccant within the evaporator and said condenser. Heat exchange means for cooling the desiccant before entering the evaporator and simultaneously heating the desiccant before entering said condenser; and.
From the first law of thermodynamics () for an adiabatic process it follows that
that is, external work is done due to changes in the internal energy of the system. Thus, an adiabatic process is the opposite of an isothermal one, since in the latter, work is performed due to the influx of an equivalent amount of heat from outside.
Means for supplying compressed hot refrigerant to said condenser and for supplying expanded cold refrigerant to the evaporator. An evaporator and a condenser, each of which has a heat and mass exchanger including tubes for receiving said refrigerant and fins attached to said tubes for receiving gravity delivered films of said liquid desiccant.
Means for circulating the desiccant and said refrigerant between and within the evaporator and said condenser. A means for removing cold dry air from the evaporator and warm moist air from said condenser; and. A means for adding moisture to said cold, dry air removed from the evaporator.
Using expressions (52.1) and (53.4), for an arbitrary gas mass we rewrite equation (55.1):
![]() . (55.2)
. (55.2)
Differentiating the equation of state for an ideal gas
![]() . (55.3)
. (55.3)
Let us exclude from (55.2) and (55.3) the temperature T:
The hybrid air conditioning system according to claim 5, wherein said adding means comprises. A humidification medium attached to said evaporator and configured in an air stream of cold dry air exhausted from the evaporator; and.
A water distributor adapted to supply water to the humidifying medium from a sump configured below the humidifying medium. A method of converting warm moist air into cold dry air, comprising stages. Circulating refrigerant and dilute liquid desiccant from the evaporator to the condenser and refrigerant and concentrated liquid desiccant from said condenser to the evaporator.
![]() .
.
Separating the variables and taking into account that (see (53.8)), we obtain
![]()
Integrating this equation over the range p 1 before p 2 and accordingly from V 1 before V 2, and then potentiating, we get
![]() , or .
, or .
The transfer of heat from a dryer before entering the evaporator to a dryer before entering said condenser. Transfer of heat and mass from warm, moist air entering the evaporator to produce cool, dry air displaced from the evaporator.
Transferring heat and mass from said condenser to air entering said condenser to produce warm moist air displaced from said condenser; and selectively humidifying said cold dry air displaced from the evaporator.
The method of converting warm moist air into cold dry air according to claim 7, wherein said transfer of heat and mass from the warm moist air stage includes removing heat and absorbing moisture from the warm moist air by placing said warm moist air in thermal contact with the cooled surface by said extended a refrigerant and placing said warm moist air in contact with a liquid desiccant flowing over said exchange surface.
Since the states 1 And 2 chosen arbitrarily, we can write
Const. (55.4)
The resulting expression is the equation gas state in an adiabatic process, also called Poisson's equation.
To go to variables T, V or r, T eliminate from (55.4) using the Clapeyron-Mendeleev equation
The method of converting warm moist air into cold dry air according to claim 7, wherein said thermal stage includes a recuperator located in said desiccant flow path for simultaneously cooling and heating the desiccant flow stream for simultaneously cooling and heating the desiccant entering the evaporator and a capacitor.
The invention relates to a vapor compression air conditioning system containing a liquid desiccant for simultaneously cooling and drying conditioned air. A liquid dehumidifier system can provide cooling when there is no active cooling by drying the air below that required for comfort, exchanging heat with the environment, and then injecting moisture into the system. However, dehumidifier systems require low wet lamp temperatures to provide the required cooling.
pressure or volume, respectively:
Const, (55.5)
Const. (55.6)
Expressions (55.4) - (55.6) represent the equations of the adiabatic process. In these equations, the dimensionless quantity (see (53.8) and (53.2))
is Poisson's ratio. For monatomic gases (Ne, He, etc.), which satisfy the ideality condition quite well, = 3, = 1.67. For diatomic gases (H 2, N 2, O 2, etc.) = 5, = 1.4. The values calculated using formula (55.7) are well confirmed by experiment.
In contrast, vapor compression systems must actively cool the air below the dew point of the air entering the evaporator to dry the air by condensation. Thus, the vapor compression system requires that the evaporator temperature be brought to a level significantly lower than that required to achieve reasonable cooling.
Hybrid liquid desiccant vapor compression systems combine the benefits of both desiccant systems with vapor compression systems. Hybrid systems combine the active, intelligent cooling found in vapor compression systems with the passive latent cooling found in dehumidifier dehumidification systems. The hybrid system does not require subcooling to remove moisture from the system. Therefore, no energy is lost due to air conditioning because moisture is absorbed rather than condensed from the air that is conditioned.
Diagram of an adiabatic process ( adiabatic) in coordinates p, V is depicted as a hyperbola (Fig. 83). The figure shows that the adiabat ( = const) is steeper than the isotherm ( pV= const). This is explained by the fact that during adiabatic compression 1-3 An increase in gas pressure is caused not only by a decrease in its volume, as with isothermal compression, but also by an increase in temperature.
Hybrid liquid-dryer vapor-compression systems work by intelligently cooling the air and absorbing moisture from the air. Sensitive cooling occurs by circulating compressed and expanded refrigerant between the evaporator and condenser found in a standard vapor compression system. Dehumidification occurs by contacting air with a desiccant on mass transfer surfaces. The mass exchange surfaces are sprayed with a liquid desiccant as outside air, air returning from the conditioned space, or a mixture of both is drawn or blown across the mass exchange surfaces.
Let us calculate the work done by the gas in an adiabatic process. Let us write equation (55.2) in the form
![]()
If a gas expands adiabatically from its volume V 1 before V 2, then its temperature drops from T 1 before T 2 and work of expansion of an ideal gas
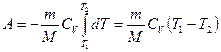 . (55.8)
. (55.8)
The mass exchange surfaces described in the prior art are separated from the heat exchange surface of the vapor compression system. Conventional mass transfer surfaces often require a separate heat transfer surface to pre-dry or pre-heat the desiccant before spraying into the mass exchanger. Problems associated with separate thermal and mass transfer surfaces are the increased costs required to purchase separate thermal and mass transfer surfaces and reduced thermal and mass transfer efficiency.
Using the same techniques as when deriving formula (55.5), expression (55.8) for work during an adiabatic process can be transformed to the form 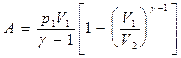
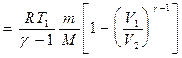
Work done by a gas during adiabatic expansion 1-2 (determined by the shaded area in Fig. 83), less than with isothermal. This is explained by the fact that during adiabatic expansion the gas is cooled, while during isothermal expansion the temperature is maintained constant due to the influx of an equivalent amount of heat from outside.
In the dehumidification process, moisture is sorbed from conditioned air by atomizing and cooling the desiccant in contact with the air in a sorbent mass exchanger or sorber. Water is sorbed in direct contact with sprayed droplets of the desiccant, entrained by air or falling films of the covering part of the desiccant or the entire surface of the mass compression of the sorber. Conventional atomization methods are ineffective methods for dehumidifying air because atomization creates an adiabatic sorption process that increases the temperature of the sorbent, thereby reducing mass transfer.
The considered isochoric, isobaric, isothermal and adiabatic processes have common feature– they occur at constant heat capacity. In the first two processes, the heat capacities are respectively equal C V and C p in an isothermal process (dT=0) the heat capacity is equal to ±∞, in an adiabatic process (δQ=0) the heat capacity is zero. The process in which the heat capacity remains constant is called polytropic.
Thus, a conventional spray agent requires more heat transfer surfaces and creates a less efficient system since refrigeration is required to remove condensation heat, solution heat, and sensitive heat transferred from the air. Conventional energy wastes from a hybrid system also by transferring heat by heat exchange means external to the heat exchanger surfaces of the vapor compression system, or by circulating a desiccant through the heat exchange surfaces of the vapor compression system.
During mass transfer, the desiccant solution is diluted with water and, under the influence of gravity, falls onto a settling tank or reservoir located inside or under the sorber. To maintain the drying process, the diluted desiccant must be desorbed, that is, regenerated. Regeneration is carried out by spraying and heating a dilute desiccant in contact with air displaced from a desorbing mass exchanger or desorber. Consequently, part of the dilute desiccant in the sorber sump is pumped into the stripper for concentration.
Based on the first law of thermodynamics, under the condition of constant heat capacity (C=const), we can derive the polytropic equation:
where is the polytropic index. Obviously, for C=0, n=γ, from (55.9) the adiabatic equation is obtained; at С=∞, n=1 – isotherm equation; at C=C p, n=0 – isobar equation, at C=C V, n=±∞ – isochore equation. Thus, all the processes considered are special cases of a polytropic process.
Water is desorbed from sprayed drops of desiccant entrained by air, or from falling films covering part of the desiccant or all surfaces of the desorber mass exchanger. Heating is required to provide the heat of vaporization necessary to evaporate water from the desiccant solution and to heat the air that comes into contact with the desiccant solution. Heat is provided by a primary energy source such as natural gas or electricity, or a renewable energy source such as solar energy, waste heat, or any combination of these sources.
In the course of studying the topic “Fundamentals of molecular kinetic theory,” we examined the so-called isoprocesses, that is, processes that occur at a constant one of the macroscopic parameters (pressure, volume or temperature). When substituting these data into the Clapeyron equation, these conditions slightly modified it. Now we will do a similar analysis, but with the first law of thermodynamics:
When waste heat from a vapor compression system is recovered, the heat is transferred by heat transfer means external to the heat exchange surfaces of the vapor compression system, or by circulating a desiccant over all heat exchange surfaces of the vapor compression system. The desiccant solution is concentrated during this process and falls by gravity into a settling tank inside or below the stripper. Continuous dehumidification is facilitated by pumping the same mass flow rate of desiccant from the desorber sump to the sorber as was sent from the sorber sump to the stripper.
Let us consider the case of the process occurring at a constant temperature:
Hence:
![]()
This means that the first law of thermodynamics takes the form:
Having considered the case of positive work being performed on a gas by external forces:
That is, during isothermal compression or expansion, the gas releases heat into the environment.
Hybrid vapor-tight liquid dryers, which recover waste heat to generate part or all of the desiccant, are more efficient systems than those that use primary energy or alternative energy for regeneration. Additionally, hybrid liquid-steam dehumidification systems that are configured for low-temperature regeneration are more efficient than systems that regenerate at higher temperatures. Conventional hybrid systems incorporating spray delivery vehicles require higher regeneration temperatures, thereby reducing the thermal efficiency of the system.
Let's consider the case when the process occurs at constant pressure:
Since no factor is included in any thermodynamic quantity, no term of the first law of thermodynamics is reset, and it retains its previous form:
Now let's consider what changes will occur in the recording of the first law of thermodynamics if the process occurs in a fixed volume.
Moreover, conventional hybrid systems that do not combine heat and mass transfer surfaces on a single surface are less efficient and require more work energy. The present invention simultaneously dehumidifies and cools air using standard vapor compression equipment and aqueous solutions liquid desiccants. The invention is a hybrid air conditioning system comprising a standard compressor, evaporator, condenser and refrigerant. In addition, liquid desiccant and refrigerant simultaneously circulate between the evaporator and condenser to cool and dry the air discharged therein.
Hence:
![]()
This means that the first law of thermodynamics takes the form:
That is, the meaning of using isochoric processes in heat engines is lost, because all the energy of the burned fuel will go to change the internal energy, and the gas will not do useful work.
There is also another very important process- adiabatic.
Definition.Adiabatic process - a process occurring in a thermally insulated system. That is, without supplying gas or releasing heat from gas.
The first law of thermodynamics takes the form:
That is, as a result of work done on the gas by external forces, it internal energy, and therefore the temperature increases, and when work is done with gas, it decreases.
The following experiments are clear examples of the last two statements.
1. A cylinder closed by a moving piston contains a small amount of fuel. After quickly pressing the piston, the fuel ignites.
2. In a container closed with a stopper and threaded with a pump hose, there is a small amount of water. After a certain amount of air is pumped into the vessel, the stopper quickly flies out and fog is observed in the vessel (Fig. 1).
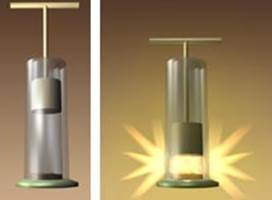

Rice. 1. Illustrative examples adiabatic processes ()
In two cases, the gas performs work of different signs, and, therefore, the change in internal energy and temperature has different signs. In both cases, the emphasis is on a rapid change in volume because it is impossible to create a perfectly isolated system, but if we consider a very transient process, then the heat will not have time to be transferred, and the process can be considered adiabatic.
Graph of adiabatic processes in P-V coordinates looks like this (Fig. 2).
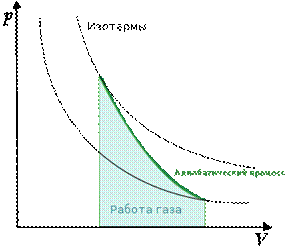
Rice. 2. Graph of the adiabatic process ()
Starting from the next lesson, we move on to studying a new section of physics - electrostatics.
Bibliography
- Myakishev G.Ya., Sinyakov A.Z. Molecular physics. Thermodynamics. - M.: Bustard, 2010.
- Gendenshtein L.E., Dick Yu.I. Physics 10th grade. - M.: Ilexa, 2005.
- Kasyanov V.A. Physics 10th grade. - M.: Bustard, 2010.
- Flash-fizika.narod.ru ().
- Youtube.com().
- Nashaucheba.ru ().
Homework
- Page 82: No. 635-639. Physics. Problem book. 10-11 grades. Rymkevich A.P. - M.: Bustard, 2013. ()
- With the adiabatic expansion of 64 g of oxygen under normal conditions, the temperature increased by 2 times. How much work has been done by the gas?
- Determine the amount of heat imparted to 2 kg of helium at a constant volume if its temperature increased by 100 K. How much did the internal energy of the gas change and how much work was done by it?
- * Why, if you knock off the valve of a compressed air cylinder, frost appears on the neck of the cylinder?
