There are three sources of amino acids in the cell - supply from the blood, breakdown of its own intracellular proteins and synthesis of non-essential amino acids.
The path of further transformation of amino acids depends on the type and function of the cell, the conditions of its existence and hormonal influences. The range of substances obtained by the cell from amino acids is extremely wide.
The reactions of transformation of amino acids in the cell are conventionally divided into three parts, depending on the reacting group:
1. along the side chain (radical):
Amino acids can provide energy
Since the body contains 20 proteinogenic and even more non-proteinogenic amino acids, which differ from each other in the structure of the side radical, there are a similar number of specific pathways for their catabolism of their side group. But, nevertheless, all these paths merge and converge to six products that enter the TCA cycle and here are completely oxidized to carbon dioxide and water with the release of energy. Of the total amount of energy produced in the body, amino acids account for about 10%.

Under certain conditions, the carbon skeleton of amino acids does not decompose, but participates in the synthesis of carbohydrates (glucogenic amino acids) and lipids (ketogenic amino acids).
Glucogenic amino acids include amino acids (the majority of them), the breakdown of which produces pyruvate and metabolites of the TCA cycle, for example, oxaloacetate or α-ketoglutarate.
Lysine and leucine are strictly ketogenic; their oxidation produces exclusively acetyl-S-CoA. It takes part in the synthesis of ketone bodies, fatty acids and cholesterol.
A small group of mixed amino acids is also isolated, from which pyruvate, metabolites of the TCA cycle and acetyl-S-CoA (phenylalanine, tyrosine, isoleucine, tryptophan) are formed.
2. for the carboxyl group:
Neurotransmitters are formed from amino acids
The synthesis of neurotransmitters from amino acids is primarily associated with the involvement of the α-carboxyl group of amino acids in metabolism or, more simply, its removal.
Histamine
The reaction of histamine formation is most active in mast cells of the lungs, skin, liver, basophils and eosinophils. In them, histamine is synthesized and accumulated in secretory granules.

Histamine synthesis reaction
Histamine is released into the blood when tissue is damaged, during an impact, or during electrical stimulation. In clinical practice, histamine secretion is usually associated with allergies - when an antigen re-enters a previously sensitized body, an allergic reaction develops.
Physiological effects
dilation of arterioles and capillaries and, as a result, redness of the skin, decreased blood pressure;
increased permeability of the capillary wall and, as a result, release of fluid into the intercellular space (edema), decreased blood pressure;
if the previous points occur in the brain - increased intracranial pressure;
increases the tone of the smooth muscles of the bronchi, resulting in spasm and suffocation;
weakly increases muscle tone of the gastrointestinal tract;
stimulates the secretion of saliva and gastric juice.
Serotonin
Serotonin is actively synthesized in mast cells of the skin, lungs, liver, spleen, and central nervous system.
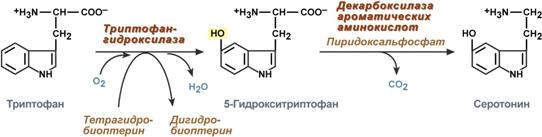
Serotonin synthesis reactions
Physiological effects
stimulates contraction of smooth muscles of the gastrointestinal tract and, as a result, increased gastrointestinal motility;
strongly stimulates the contraction of vascular smooth muscles, except for myocardial and skeletal muscle vessels and, as a result, an increase in blood pressure;
weakly increases the tone of bronchial smooth muscles;
in the central nervous system is an inhibitory neurotransmitter;
in peripheral nerve endings causes pain and itching (for example, with an insect bite).
Gamma-aminobutyric acid
The synthesis of γ-aminobutyric acid (GABA) occurs exclusively in the central nervous system - in the subcortical formations of the brain.

GABA synthesis reaction
Physiological effects
In the central nervous system, GABA (along with glutamic acid) is an inhibitory transmitter. Its role is highest in the temporal and frontal cortex, hippocampus, amygdala and hypothalamic nuclei, substantia nigra, and cerebellar nuclei.
Dopamine synthesis occurs mainly in the neurons of the diencephalon and midbrain.

Dopamine synthesis reactions
Physiological effects
It is a mediator of dopamine receptors in the subcortical formations of the central nervous system; in large doses, it dilates the vessels of the heart, stimulates the frequency and strength of heart contractions, dilates the vessels of the kidneys, increasing diuresis.
Neutralization biogenic amines
There are two types of reactions inactivating biogenic amines – deamination and methylation.
Deamination occurs with the formation of free ammonia and with the participation of FAD. The reaction is catalyzed by monoamine oxidase; it is found in many tissues, but is most active in the liver, stomach, kidneys, intestines, and nervous tissue.
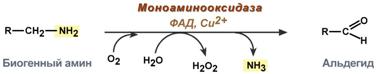
Reaction involving monoamine oxidase
Methylation of a biogenic amine occurs when it has a hydroxyl group (dopamine, serotonin). The active form of methionine, S-adenosylmethionine (SAM), takes part in the reaction, producing the methylated form of the amine and S-adenosylhomocysteine (SAH).
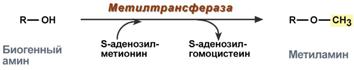
Methylation reaction
3. involving an amino group:
There are 4 types of deamination
The transformation of amino acids with the participation of the NH2 group is reduced to its elimination from the carbon skeleton - the deamination reaction.
Types of deamination
intramolecular – with the formation of unsaturated fatty acid:

reducing – with the formation of saturated fatty acid:
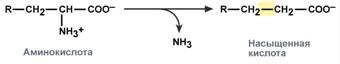
hydrolytic – with the formation of carboxylic hydroxy acid:
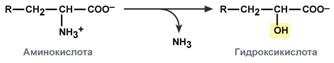
oxidative – with the formation of keto acids:

In humans, oxidative deamination is the main pathway of amino acid catabolism. However, amino acids such as serine and histidine can lose their amino group using other types of deamination, and threonine undergoes direct cleavage to glycine and acetaldehyde.
Amino acids must be transported across membranes
The transfer of amino acids across cell membranes, both during absorption from the intestinal cavity into enterocytes and during passage from the blood into the cells of various tissues, is carried out using two mechanisms: secondary active transport and the glutathione transport system.
Transport of amino acids across membranes
Secondary active transport
Secondary active transport is the transfer of substances, in this case amino acids, using a sodium concentration gradient between the inner and outer sides of the cell membrane.
Secondary active transport is based on the use of a low concentration of sodium ions inside cells created by the membrane enzyme Na+,K+-ATPase. A specific transporter protein binds amino acid and sodium ion on the apical surface of enterocytes. The important thing is that in the absence of sodium, the amino acid is not able to bind to the carrier protein.
Then, changing its position in the membrane, the protein releases sodium ion into the cytosol along a concentration gradient. Immediately after this, the amino acid loses its connection with the protein and remains in the cytoplasm.
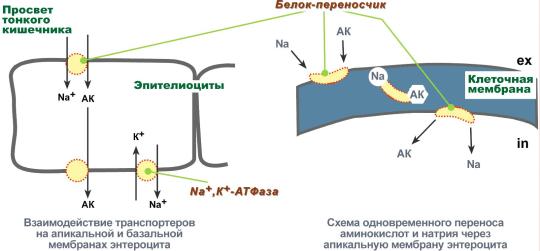
Secondary active transport of amino acids across membranes
Currently there are 5 transport systems:
for large neutral, including aliphatic and aromatic amino acids,
for small neutrals - alanine, serine, threonine,
for basic amino acids – arginine and lysine,
for acidic amino acids – aspartate and glutamate,
for small amino acids - glycine, proline and hydroxyproline.
Glutathione transport system
The second method of transferring amino acids into the cell occurs in combination with glutathione using the enzyme γ-glutamyltransferase.
Transport of amino acids with the participation of glutathione

The carrier of some amino acids (usually neutral) according to this scheme is the tripeptide glutathione (γ-glutamylcysteylglycine). When glutathione interacts with an amino acid on the outside of the cell membrane with the participation of glutamyl transferase. The γ-glutamyl residue binds the amino acid and moves it into the cell. Glutathione breaks down into its components. After the amino acid is separated, glutathione is resynthesized.
Ammonia is constantly produced in cells
Ammonia is continuously formed in all organs and tissues of the body. Its most active producers into the blood are organs with a high metabolism of amino acids and biogenic amines - nervous tissue, liver, intestines, muscles.
Main sources of ammonia
The main sources of ammonia are the following reactions:
non-oxidative deamination of some amino acids (serine, threonine, histidine) - in the liver,
oxidative deamination of glutamic acid in all tissues (except muscle), especially in the liver and kidneys,
deamination of amides of glutamic and aspartic acids – in the liver and kidneys,
catabolism of biogenic amines - in all tissues, to the greatest extent in nervous tissue,
vital activity of bacteria in the large intestine,
breakdown of purine and pyrimidine bases - in all tissues.
Ammonia fixation
Since ammonia is an extremely toxic compound, several reactions of ammonia binding (neutralization) exist in tissues - the synthesis of glutamic acid and glutamine, the synthesis of asparagine, the synthesis of carbamoyl phosphate:
synthesis of glutamic acid (reductive amination) - interaction of α-ketoglutarate with ammonia. The reaction is essentially the reverse of oxidative deamination, but uses NADPH as a coenzyme. Occurs in almost all tissues except muscle, but is of little significance, because for glutamate dehydrogenase the preferred substrate is glutamic acid and the reaction equilibrium is shifted towards α-ketoglutarate,
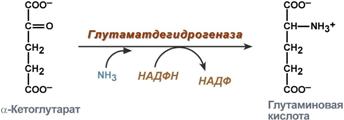
Glutamic acid synthesis reaction
glutamine synthesis - the interaction of glutamate with ammonia. It is the main method of removing ammonia; it occurs most actively in nervous and muscle tissues, in the kidneys, retina, and liver. The reaction takes place in mitochondria.
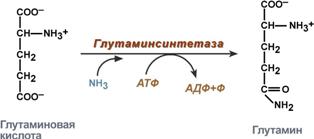
Glutamine synthesis reaction
The formation of a large amount of glutamine ensures high concentrations in the blood (0.5-0.7 mmol/l).
Since glutamine penetrates cell membranes by facilitated diffusion, it easily enters not only hepatocytes, but also other cells where there is a need for amino groups. The nitrogen carried by glutamine is used by cells to synthesize purine and pyrimidine rings, guanosine monophosphate (GMP), asparagine, and glucosamine-6-phosphate (the precursor to all other amino sugars).
synthesis of asparagine - interaction of aspartate with ammonia. It is a secondary method of ammonia removal; it is energetically disadvantageous, because in this case, 2 macroergic connections are wasted,
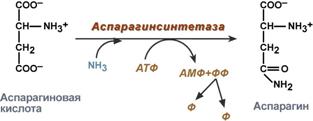
Asparagine synthesis reaction
synthesis of carbamoyl phosphate in liver mitochondria - the reaction is the first in the process of synthesis of urea, a means of removing ammonia from the body.
Ammonia transport
The transport forms of ammonia from tissues to the liver are glutamine and alanine, and to a lesser extent asparagine and glutamate; some ammonia is found in the blood in free form. Glutamine and alanine are the most represented, their share among all blood amino acids is up to 50%. Most of glutamine comes from muscles and nerve tissue, alanine carries ammonia from muscles and the intestinal wall.
Glucose-alanine cycle
In muscles, the main acceptor of excess amine nitrogen is pyruvate. During protein catabolism in muscles, amino acid transamination reactions occur, glutamate is formed, which then transfers amino nitrogen to pyruvate and alanine is formed. From the muscles in the blood, alanine is transported to the liver, where in a reverse reaction it transfers its amino group to glutamate. The resulting pyruvate is used as a substrate in the reactions of glucose synthesis (gluconeogenesis), and glutamic acid is deaminated and ammonia is used in the synthesis of urea.
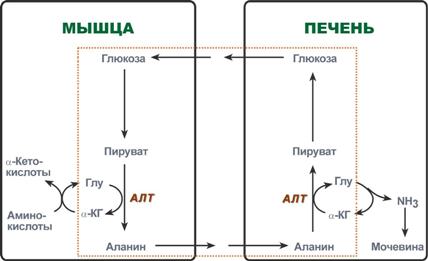
Reactions of the glucose-alanine cycle (highlighted by a frame).
Reactions associated with transport forms of ammonia
The target organs for ammonia transport are the liver, kidneys and intestines.
In the liver:
asparagine and glutamine are deaminated by asparaginase and glutaminase, respectively, the resulting ammonia is used for the synthesis of urea,
Alanine undergoes transamination reactions with α-ketoglutarate,
Glutamic acid undergoes oxidative deamination.
In the intestine, part of the glutamine is deaminated by glutaminase. After this, the formed ammonia is released into the intestinal lumen (no more than 5%) or through the blood of the portal vein goes to the liver, and glutamate enters into transamination with pyruvate, as a result of which amino nitrogen passes to alanine and also enters the liver with it,
In the kidneys, ammonium salts are formed using glutamate, glutamine and asparagine.
Ammonia accumulation is a problem
Ammonia is a toxic compound found in the blood in relatively small concentrations (11.0-32.0 µmol/l). Symptoms of ammonia poisoning appear when these limits are exceeded by only 2-3 times. The maximum permissible level of ammonia in the blood is 60 µmol/l. When ammonia concentrations increase (hyperammonemia) to extreme values, coma and death can occur. With chronic hyperammonemia, mental retardation develops.
Ammonia toxicity hypotheses
The toxicity of ammonia is due to the following circumstances:
1. The binding of ammonia during glutamate synthesis causes an outflow of α-ketoglutarate from the tricarboxylic acid cycle, which reduces the production of ATP energy and impairs cell activity.
2. Ammonium ions NH4+ cause alkalization of blood plasma. At the same time, the affinity of hemoglobin for oxygen increases (Bohr effect), hemoglobin does not release oxygen in the capillaries, resulting in cell hypoxia.
3. The accumulation of free NH4+ ion in the cytosol affects the membrane potential and the work of intracellular enzymes - it competes with ion pumps for Na+ and K+.
4. The product of ammonia binding to glutamic acid - glutamine - is osmotically active substance. This leads to water retention in the cells and their swelling, which causes tissue swelling. In the case of nerve tissue, this can cause brain swelling, coma and death.
5. The use of α-ketoglutarate and glutamate to neutralize ammonia causes a decrease in the synthesis of γ-aminobutyric acid (GABA), an inhibitory neurotransmitter of the nervous system.
Hereditary and acquired forms of hyperammonemia
Acquired forms
Acquired (secondary) hyperammonemia develops as a result of liver diseases and viral infections. In extremely severe cases, it manifests itself as nausea, vomiting, convulsions, slurred speech, blurred vision, tremors, and impaired coordination of movements.
Hereditary forms
Hereditary forms of hyperammonemia are caused by a genetic defect in any of the five urea synthesis enzymes. According to the enzyme, the disease is divided into five types. The primary signs of hyperammonemia are drowsiness, refusal to eat, vomiting, anxiety, convulsions, impaired coordination of movements, tachypnea, and respiratory alkalosis. Liver failure, pulmonary and intracranial hemorrhages may develop.
The most common is hyperammonemia type II, associated with a deficiency of ornithine carbamoyltransferase. The disease is recessive, linked to the X chromosome. The mother also has hyperammonemia and an aversion to protein foods. With a complete enzyme defect, hereditary hyperammonemia has an early onset (up to 48 hours after birth).
A laboratory criterion for the disease is the accumulation of glutamine (20 times or more) and ammonia in the blood, cerebrospinal fluid and urine.
The basis of treatment for hyperammonemia comes down to limiting protein in the diet; this alone can prevent many disorders of brain activity.
Urea synthesis
In the liver, all removed ammonia is used for the synthesis of urea. An increase in urea synthesis is observed during the breakdown of tissue proteins and nitrogenous compounds (fasting, inflammatory processes, diabetes mellitus) or with excess protein nutrition. In infants and children, urea synthesis can be reduced for two reasons: liver immaturity and active synthesis of proteins and nucleic acids during body growth.
The reactions of urea synthesis are a cyclic process and are called the ornithine cycle. Urea synthesis begins in mitochondria (first and second reactions), the remaining three reactions occur in the cytosol. Special transporters exist to transport citrulline and ornithine across the mitochondrial membrane.
Fumaric acid is produced as a by-product of the ornithine cycle and is transported back into the mitochondria. Here, in the reactions of the TCA cycle, oxaloacetate is formed from it, which is transaminated with glutamate to aspartate, enters the cytosol and reacts again with citrulline.
The formation of one urea molecule involves 1 NH4+ molecule, 1 CO2 molecule, the amino group of 1 aspartic acid molecule, and 4 high-energy bonds of three ATP molecules.

Creatine phosphate - urgent energy reserve
Creatine is a substance of skeletal muscles, myocardium, and nervous tissue. In the form of creatine phosphate, creatine is a “depot” of macroergic bonds and is used for rapid resynthesis of ATP during cell work.

Use of creatine phosphate for ATP resynthesis
The role of creatine in muscle tissue. Creatine phosphate ensures ATP resynthesis in the first seconds of work (5-10 sec), when neither anaerobic glycolysis nor aerobic oxidation of glucose and fatty acids is yet activated, and the blood supply to the muscle is not increased. In nervous tissue cells, creatine phosphate maintains cell viability in the absence of oxygen.
During muscular work, Ca2+ ions released from the sarcoplasmic reticulum are activators of creatine kinase. The reaction is also interesting because in its example one can observe a positive feedback relationship - activation of the enzyme by the reaction product creatine. This avoids a decrease in the reaction rate during work, which would occur according to the law of mass action due to a decrease in the concentration of creatine phosphate in the working muscles.
About 3% of creatine phosphate is constantly converted into creatinine in a non-enzymatic dephosphorylation reaction. The amount of creatinine secreted by a healthy person per day is always almost the same and depends only on the volume of muscle mass.

Formation of creatinine from creatine phosphate
Creatine synthesis occurs sequentially in the kidneys and liver in two transferase reactions. After synthesis is completed, creatine is delivered through the bloodstream to the muscles or brain.


Reactions of creatine synthesis in the kidneys and liver
Here, in the presence of ATP energy (during rest or rest), it is phosphorylated to form creatine phosphate.
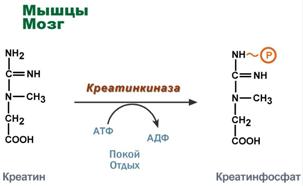
Creatine phosphate synthesis
If the synthesis of creatine outstrips the possibility of its fixation in muscle tissue, then creatinuria develops - the appearance of creatine in the urine. Physiological creatinuria is observed in the first years of a child’s life. Sometimes creatinuria in old people is classified as physiological, which occurs as a result of muscle atrophy and incomplete use of creatine formed in the liver. In diseases of the muscular system (myopathy or progressive muscular dystrophy), the highest concentrations of creatine are observed in the urine - pathological creatinuria.
Literature
Pathways for the biosynthesis of protein-forming amino acids ( proteinogenic) are quite complex, multifaceted (the same amino acid can be synthesized different ways) and can differ significantly among different organisms. Nevertheless, there are quite a large number of patterns in these processes, and for convenience, all 20 proteinogenic amino acids can be divided into 5 biosynthetic families. Amino acids belonging to the same family are characterized by the presence of common precursors that are formed in the TCA cycle, during glycolysis, and during the pentose phosphate pathway.
Nonessential amino acids are synthesized using quite simple reactions, while the biosynthesis pathways of essential amino acids are very complex. Essential amino acids for white rats include: valine, isoleucine, leucine, threonine, methionine, lysine, phenylalanine, tryptophan, histidine and arginine. Eight of the ten listed amino acids are also not synthesized by the human body; whether histidine and arginine are essential for humans remains controversial.
Biosynthesis of amino acids of the glutamate family . This family includes: glutamate, glutamine, proline, and arginine. The first two amino acids are formed from a-ketoglutarate, and the amino groups originate from ammonia molecules (Fig. 16.3). Proline is synthesized from glutamate through four reactions: The g-carboxyl group of glutamate reacts with ATP to form an acyl phosphate. The latter is reduced with the participation of NADPH to an aldehyde, and then, during spontaneous dehydration, is converted into a cyclic compound - pyrroline carboxylate. This product is reduced with the participation of NADPH to proline (Fig. 16.4).
The synthesis of arginine (Fig. 16.5) is also carried out from glutamate, which is first acetylated at the amino group, and then undergoes the phosphorylation reactions already described above and the formation of semialdehyde. However, the g-semialdehyde N-acetylglutamate does not cyclize, as in the proline biosynthesis pathway, but is transaminated with the participation of glutamate (Glu). As a result of this reaction, a-ketoglutarate (KG) and N-acetylornithine are formed. The latter undergoes deacetylation to form ornithine. Ornithine is converted to arginine through several reactions represented in the urea cycle.
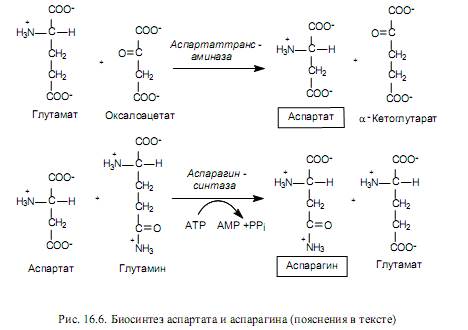
Biosynthesis of amino acids of the aspartate family. The aspartate family includes: aspartate, asparagine, lysine, threonine, isoleucine and methionine. The last five amino acids of this composition are synthesized from aspartate, which, in turn, is formed from oxaloacetate, an intermediate product of the TCA cycle, during the transamination reaction. The amino group donor in this case is glutamate (Fig. 16.6).
Aspartate serves as a precursor for the synthesis of asparagine, and in many bacteria direct amination of aspartate can occur in an ATP-dependent reaction involving asparagine synthetase (16.2).
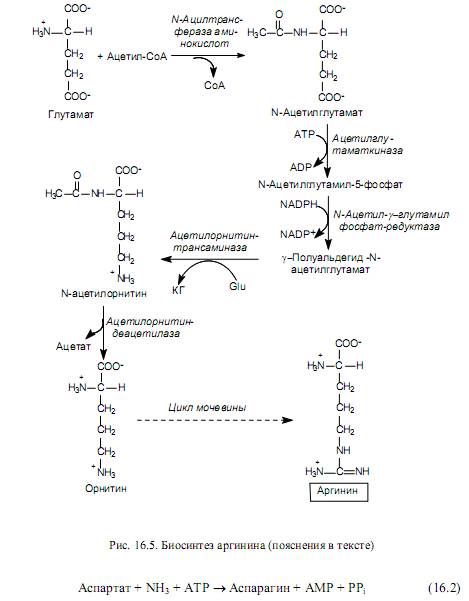
In mammalian cells, another reaction occurs (Fig. 16.6), in which glutamine acts as a donor of the amino group during the formation of asparagine.
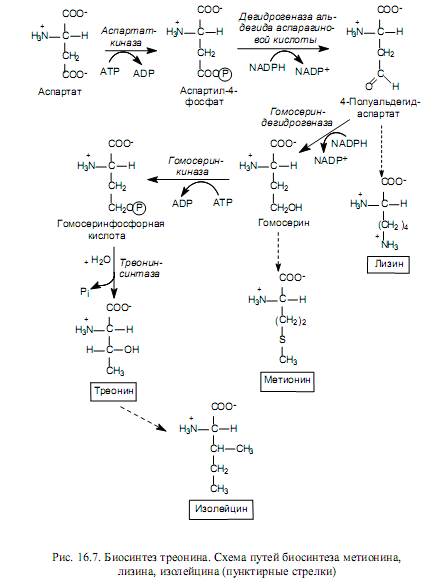
Lysine, methionine and threonine are synthesized from aspartate derivatives (Fig. 16.7), and isoleucine is synthesized from threonine.
Lysine in bacterial and plant cells is synthesized during the aldol condensation of aspartic acid semialdehyde and pyruvate, followed by reduction, addition of a succinate residue, transamination with the participation of glutamate, intramolecular rearrangement and decarboxylation. Fungal cells use another pathway for lysine biosynthesis - from a-ketoglutarate and acetyl-CoA.
The carbon skeleton of methionine is formed from homoserine, the sulfur atom comes from cysteine, and the methyl group donor is N-methyltetrahydrofolic acid.
Threonine provides four of the six carbon atoms in the isoleucine molecule. At the first stage of synthesis, threonine is deaminated, turning into 2-ketobutyrate, then interacts with pyruvate, undergoes structural rearrangements and a transamination reaction, in which glutamate acts as a donor of the amino group.
Biosynthesis of amino acids of the pyruvate family. From pyruvate the following are synthesized: alanine, valine and leucine.
Alanine is formed in a transamination reaction, where glutamate serves as an amino group donor (Fig. 16.8). The synthesis of valine and leucine has several common stages and begins with the formation of acetolactate. This metabolite is formed from two molecules of pyruvate: one of them is decarboxylated, and the resulting active acetate is transferred to the second molecule (Fig. 16.8). This reaction is catalyzed by acetolactate synthase with the participation of thiamine pyrophosphate. 2-Acetolactate is reduced to dihydroxyisovaleric acid, which is accompanied by migration of the methyl group. Dioxyisovalerate dehydrates to 2-ketoisovalerate. This product can be converted to valine in a transamination reaction involving glutamate, and can also condense with acetyl-CoA and, through several reactions (isomerization, reduction, decarboxylation, transamination), be converted to leucine. Glutamate is also a donor of the amino group in the formation of leucine (Fig. 16.8).
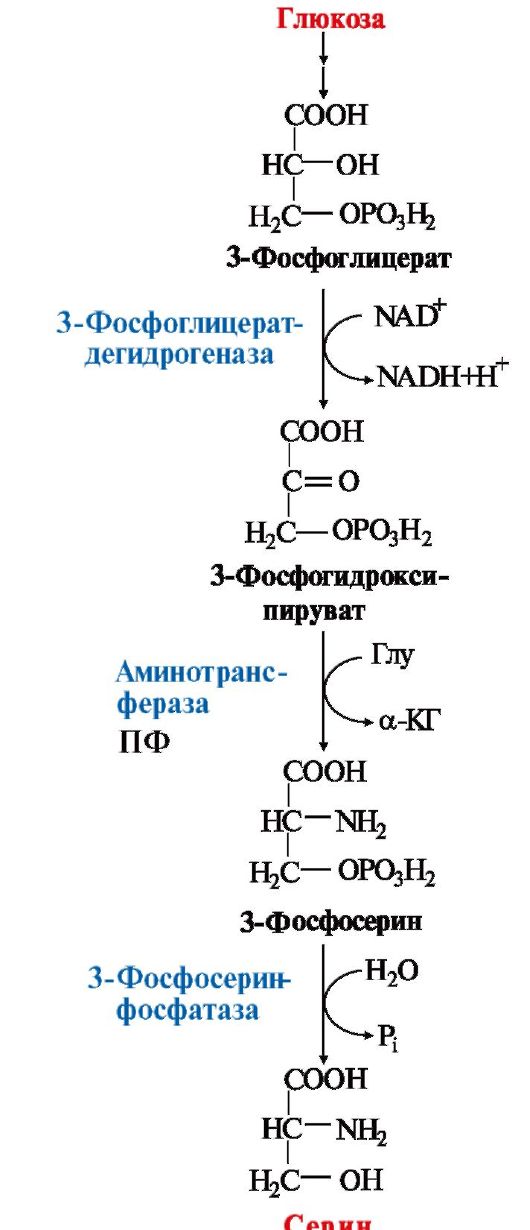
Biosynthesis of amino acids of the serine family. The family includes serine, cysteine and glycine. The precursor of these amino acids is 3-phosphoglycerate, an intermediate product of glycolysis.
3-Phosphoglycerate is oxidized to 3-phosphohydroxypyruvate and then aminated with glutamate to 3-phosphoserine and dephosphorylated to serine (Fig. 16.9). There is also alternative path, when the elimination of the phosphate group occurs before the oxidation reaction:
3-Phosphoglycerate→Glycerate→Hydroxypyruvate→Serine
Serine serves as a substrate for the synthesis of glycine and cysteine. During the formation of glycine, the b-carbon atom of the serine side chain is accepted by the transporter of one-carbon fragments - the cofactor tetrahydrofolic acid with the participation of the enzyme serine hydroxymethyl transferase (Fig. 16.9). There is another route for the synthesis of glycine: from CO 2, NH + 4 and methylenetetrahydrofolic acid, which is catalyzed by glycine synthase.

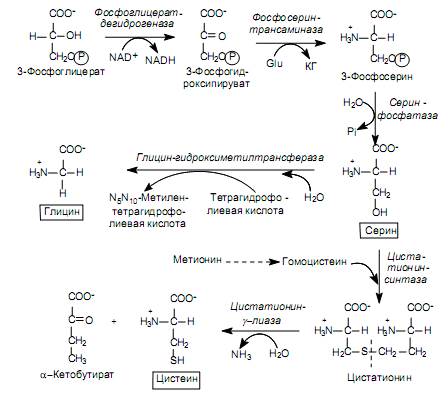
The conversion of serine to cysteine is associated with the replacement of the side chain oxygen atom with a sulfur atom, the donor of which is methionine. First, methionine, in a series of ATP-dependent reactions, where its activated form (S-adenosylmethionine) is formed, loses the methyl group at the sulfur atom and turns into homocysteine:
Homocysteine then reacts with serine to form cystathionine, which is cleaved by cystathionine g-lyase into cysteine and a-ketobutyrate (Fig. 16.9).
Some microorganisms have an alternative pathway for the synthesis of cysteine, where hydrogen sulfide serves as the donor of the sulfur atom. In this case, serine is first acetylated by acetyl-CoA (the reaction is catalyzed by serine transacetylase), and then acetylserine reacts with hydrogen sulfide with the participation of O-acetylserine sulfhydrolase:
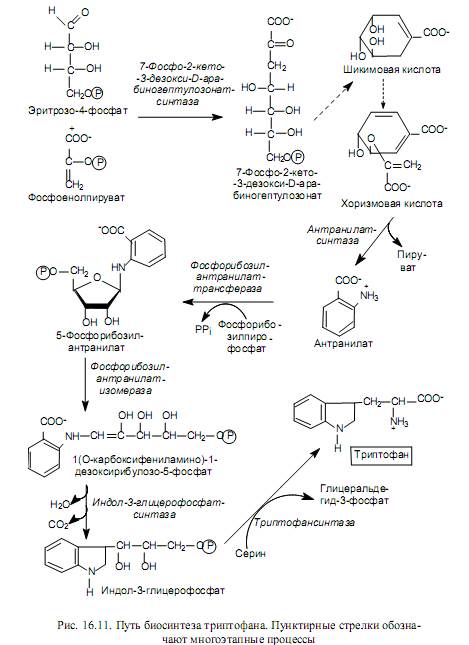
Biosynthesis of amino acids of the pentose family. The amino acids belonging to this family (histidine, tryptophan, phenylalanine and tyrosine) are synthesized with the participation of the five-carbon intermediate of the pentose phosphate pathways - ribose-5-phosphate, on the basis of which they are grouped into the pentose family. In Fig. Figure 16.10 shows the pathways for the transformation of ribose-5-phosphate, leading to the formation of compounds from which the named amino acids are synthesized.
The process of histidine biosynthesis is quite complex and involves 5-phosphoribosyl-1-pyrophosphate, ATP and glutamine. In Fig. Figure 16.10 in the composition of the histidine molecule shows the origin of the carbon and nitrogen atoms: one nitrogen atom of the imidazole ring comes from the amide group of glutamine, the other nitrogen atom and one of the carbon atoms of the ring originate from ATP, and the remaining carbon atoms originate from 5-phosphoribosyl-1- pyrophosphate.
The biosynthesis of aromatic amino acids begins with the stage of condensation of erythrose-4-phosphate with phosphoenolpyruvate. The resulting seven-carbon compound (7-phospho-2-keto-3-deoxy-D-arabinoheptulosonate) is dephosphorylated, cyclized, dehydrated, and reduced with the participation of NADPH to shikimic acid. Shikimic acid undergoes another condensation with phosphoenolpyruvate and, after elimination of the phosphoric acid residue, is converted to chorismic acid (Fig. 16.11). Chorismate serves as a major precursor to the tryptophan biosynthesis pathway, which is depicted in Fig. 16.11.
Chorismic acid is also used for the synthesis of phenylalanine, i.e. at the stage of its formation, the biosynthesis pathways of two essential aromatic amino acids - tryptophan and phenylalanine - diverge (hence the name chorismate, which comes from the Greek word meaning “fork”).
Phenylalanine is formed through three sequential reactions: isomerization of chorismate to prephenate, dehydration and decarboxylation of prephenate to phenylpyruvate, and transamination of phenylpyruvate with the participation of glutamate.
The non-essential acid tyrosine can be synthesized from phenylalanine by its hydroxylation with the participation of phenylalanine 4-monooxygenase, as well as from prefenic acid after its decarboxylation and amination.

Patterns of amino acid biosynthesis . A review of the biosynthesis pathways of proteinogenic amino acids allows us to identify the following basic patterns of these processes: 1) the carbon skeletons of amino acids originate from intermediate products of glycolysis (3-phosphoglycerate, phosphoenolpyruvate, pyruvate), pentose phosphate pathways (ribose-5-phosphate and erythrose-4-phosphate) , TCA cycle (oxaloacetate and a-ketoglutarate); 2) the donor of amino groups for most proteinogenic amino acids is glutamate, less often glutamine; reactions in which the amino group of an amino acid is transferred to a keto acid are called “transamination reactions”; 3) the biosynthesis of many amino acids is carried out by “families” for which common precursors are used; many amino acids themselves serve as substrates for the synthesis of other amino acids; 4) many stages of amino acid biosynthesis require an influx of energy and are accompanied by ATP hydrolysis (stages of the synthesis of histidine, proline, methionine, asparagine, glutamine, arginine); in addition, the energy of activated molecules involved in synthesis is used; finally, intermediate products that could provide the cell with energy storage are removed from catabolic and amphibolic processes; 5) many stages of amino acid biosynthesis require the participation of reducing equivalents (NADH and NADPH), which could be oxidized in the respiratory chain and lead to energy gain.
Thus, the biosynthesis of amino acids is quite expensive for the cell. It is not surprising, therefore, that this process in every organism (cell) is subject to very complex regulation (Chapter 19), which, on the one hand, is determined by the complexity and branching of the biosynthesis of proteinogenic amino acids itself, and on the other hand, should ensure strict economy of cellular resources (energy, reduction equivalents, building blocks). It also seems logical that in the presence of exogenous amino acids, microbial cells, in particular, do not synthesize them independently, but use ready-made forms.
1. Carbon skeleton of the eight nonessential amino acids (Ala, Asp, Asn, Ser, Gli, Pro, Glu, Gln) And cysteine can be synthesized from glucose (Fig. 9.15).
The α-amino group is introduced into the corresponding α-keto acids via a transamination reaction. The universal donor of the α-amino group is glutamate.
Directly through transamination of OPA metabolites with glutamate, the following are synthesized:
 Rice. 9.15. Pathways for the biosynthesis of non-essential amino acids
Rice. 9.15. Pathways for the biosynthesis of non-essential amino acids
2. Partially replaceable amino acids Arg and His are synthesized in small quantities that do not meet the body's needs, which is especially noticeable in childhood. Arginine synthesis occurs in reactions of the ornithine cycle. Histidine synthesized from ATP and ribose.
Conditionally essential amino acids Tyr and Cys are formed using essential amino acids:
Phenylalanine is converted to tyrosine under the influence of phenylalanine hydroxylase;
For education cysteine sulfur is required, the donor of which is methionine. The synthesis uses the carbon skeleton and α-amino group of serine.
QUANTITATIVE CHARACTERISTICS OF AMINO ACIDS METABOLISM STUDYED IN THIS MODULAR UNIT
Serum ammonia concentration: 0.04-0.07 mg/dl (25-40 µmol/l)
Serum urea concentration: 15-50 mg/dl (2.5-8.4 mmol/l)
Daily urea excretion: -25 g/day
Daily excretion of ammonium salts: -0.5 g/day
Modular unit 3 FEATURES OF THE METABOLISM OF INDIVIDUAL AMINO ACIDS: SERIINE, GLYCINE, METHIONINE, PHENYLALANINE, TYROSINE AND HISTIDINE. ROLE OF VITAMINS B 12, B 6 AND FOLIC ACID. DISEASES ASSOCIATED WITH DISORDERS IN THE METABOLISM OF PHENYLALANINE AND TYROSINE.
SYNTHESIS, BIOLOGICAL ROLE AND INACTIVATION
BIOGENIC AMINES
TOPIC 9.9. METABOLISM OF SERINE AND GLYCINE.
ROLE OF FOLIC ACID
In addition to the metabolic pathways characteristic of most amino acids that make up proteins, there are also specific transformation pathways for almost all amino acids. Let's consider the metabolism of some amino acids, the specific pathways of transformation of which lead to the synthesis of biologically important products and largely determine the physiological state of a person.
1. Serin- a non-essential amino acid, synthesized from an intermediate
glycolysis product - 3-phosphoglycerate in the sequence of reactions of dehydrogenation, transamination and hydrolysis under the action of phosphatase
In the body, serine is used to synthesize:
Phospholipids (phosphatidylserines, sphingomyelins);
Amino acids (glycine, cysteine).
Major pathway of serine catabolism- its deamination with the formation of pyruvate (see topic 9.3).
2. Glycine is formed from serine by the action of serine oxymethyltransferase. Coenzyme this enzyme is tetrahydrofolic acid (H4-folate),
which adds the β-carbon atom of serine, forming methylene - H4-folate
Glycine is a precursor to:
Porphyrins (heme),
Purine bases
Coenzymes,
Glutathione, etc. Glycine catabolism is happening
also with with the participation of H 4 -folate, which binds the a-CH 2 group of glycine (see Fig. 9.18).
3. H 4 -folate is formed in the liver from folic acid (folate) with the participation of the enzymes folate reductase and dihydrofolate reductase (Fig. 9.19). The coenzyme of these reductases is NADPH.
Methylene group - CH 2 - in a molecule methylene-H 4 -folate can transform into other one-carbon groups:
H 4 -folate is able to transfer these groups to other compounds and plays a role intermediate carrier of one-carbon groups.
One-carbon fragments are used to synthesize nucleotides and a number of compounds (see Fig. 9.18).
 Rice. 9.17. Synthesis of serine from glucose
Rice. 9.17. Synthesis of serine from glucose
 Rice. 9.18. Biological role one-carbon groups
Rice. 9.18. Biological role one-carbon groups
Rice. 9.19. Scheme of synthesis of H 4 -folate in the liver
4. Folic acid is a vitamin for humans and most mammals (vitamin B C or AT 9). It is widely distributed in foods and is synthesized by intestinal bacteria. Hypovitaminosis It occurs quite rarely in humans. The reasons for this may be:
Poor nutrition - insufficient consumption of vegetables, fruits and meat products;
Impaired absorption of folic acid in the intestine;
Hepatitis, cirrhosis and other liver lesions that cause a decrease in folate reductase activity.
Hypovitaminosis of folic acid leads to disruption of synthesis nucleic acids in the body, which affects primarily the rapidly dividing blood cells and the development megaloblastic anemia.
5. Many pathogenic microorganisms are able to synthesize folic acid from para-aminobenzoic acid, which is a component of folate. Based on this bacteriostatic effect of sulfonamide drugs, which are structural analogues of n-aminobenzoic acid:
The drugs are competitive inhibitors of folic acid synthesis enzymes in bacteria or can be used as pseudosubstrates, resulting in the formation of a compound that does not perform the function of folic acid. This makes cell division impossible, bacteria stop multiplying and die. Sulfonamides are called antivitamins.
Introduction
2. PRODUCERS OF AMINO ACIDS.
3. BIOSYNTHESIS OF AMINO ACIDS.
3.1 One-step method for obtaining amino acids.
3.2 Two-step method for obtaining amino acids.
3.3 Obtaining lysine.
3.4 Production of amino acids using immobilized enzymes and cells.
3.5 Technology for producing glutamate.
4. INDUSTRIAL SYNTHESIS OF AMINO ACIDS.
4.1 Microbiological synthesis.
4.2 Chemical synthesis.
5. APPLICATION OF AMINO ACIDS. Conclusion.
List of sources used.
Introduction
The current level of development of biotechnology is due to the general progress of science and technology, especially over the past 50 years. It is enough to note only such events as the establishment of the structure and functions of nucleic acids, the discovery of DNA restriction enzymes and the identification of their significance in the life of cells with subsequent use in genetic engineering work, the creation of hybridomas and the production of monoclonal antibodies, the introduction of computers and computer technology into biotechnological processes.
Industrial biosynthesis of amino acids belongs to microbiotechnology. In essence, microbiotechnology is identical to industrial (technical) microbiology. Its objects are microbes - viruses (including viroids and phages), bacteria, fungi, lichens, protozoa. In some cases, biological objects are primary metabolites of microbial origin - enzymes, the catalytic activity of which underlies engineering enzymology.
Compared to plant and animal cells, microbes, as a rule, multiply faster and, therefore, all metabolic processes occur faster in them. The relative advantages of most microbes as biological objects are as follows:
1) greater “simplicity” of genome organization,
2) fairly easy adaptability (lability) to the environment in natural and artificial conditions,
3) expressed rates of enzymatic reactions and increase in cell mass per unit time.
The first advantage provides microbial cells with better opportunities for measurement and restructuring hereditary material, for example, the inclusion of foreign genetic information, the introduction of plasmids into cells or, conversely, the elimination of plasmids from them.
The second advantage associated with the lability of microbes can be illustrated by the example of bacteria and fungi. Thus, with regard to temperature, microbes are divided into psychophiles, mesophylls and thermophiles.
1. CHARACTERISTICS OF AMINO ACIDS.
Amino acids play an important role in healthcare, livestock farming and light industry. According to their importance for the macroorganism, amino acids are divided into replaceable and essential. Essential amino acids are those amino acids that are not synthesized in animals or human body, they must be brought with food or animal feed (Table 1).
Table 1
Replaceable and essential amino acids.
Nonessential ones are synthesized in vivo from ammonia and various carbon sources. Microorganisms themselves synthesize all the amino acids they need from ammonia and nitrates, and carbon “skeletons” from the corresponding intermediates.
Based on the evaluation of amino acids, scientists have long sought to exploit the ability of microorganisms to produce non-essential and essential amino acids in measurable quantities.
People's need for amino acids is quite high and this determines the level of their production in the world (about 500 thousand tons per year).
Most microorganisms and green plants are capable of synthesizing denovo all twenty amino acids. The carbon skeletons of amino acids are formed from intermediate metabolic products.
The starting material for the synthesis of amino acids are simple intermediate products of catabolism (pyruvate, 2-oxyglutarate, oxaloacetate and fumarate, erythrose-4-phosphate, ribose-5-phosphate and ATP). In the synthesis of most amino acids, the amino group is introduced only at the last stage by transamination. Some amino acids are formed by a series of transformations of other amino acids, and in these cases transamination is not required.
Proteins are synthesized on ribosomes from amino acids according to information from m-RNA, which is transcribed (by transcription) from DNA genes.
2. AMINO ACIDS PRODUCERS
Specific enzymes that regulate the biosynthesis of amino acids are widespread in bacteria; they have been studied in some depth in Escherichia coli. Salmonellatyphimurium, Bacillus subtilis and others. In fungi, due to amino acid limitation, there is an uncoordinated, parallel increase in the level of enzymes that catalyze the biosynthesis reactions of various amino acids. This "common control of amino acid biosynthesis" has also been called "metabolic interblock" or "cross-pathway regulation", first identified in Neurosporacrassa in 1965 by M. Karsiotis and co-workers, and later in Saccharomycescerevisiae, Aspergillusnidulas and other fungi.
Feedak - repression, for example, during the biosynthesis of aromatic amino acids in the last stages, plays an important role in the hyperproduction of individual amino acids by cultures of Escherichia coli, Serratia marcescens and others.
In any living organism, amino acids are spent primarily on the biosynthesis of primary metaolites - enzymatic and non-enzymatic proteins. Therefore, in addition to denovo biosynthesis of amino acids, another way of obtaining them is possible, namely from hydrolysates of the corresponding proteins (tryptophan is destroyed by acid hydrolysis), including from the native biomass of microbial cells.
Natural amino acids are, as a rule, optically active L - and D - forms, which are difficult to separate. That is why microbial synthesis with the help of corynebacteria and some other microbes is now basic and economically profitable. The first place here is rightfully occupied by Japan, where glutamic acid alone produces over 100 thousand tons per year; Most of the natural essential amino acids are produced by Takeda. S. Kinoshita, who first discovered and proved the promise of microbial synthesis in the 50s, already admitted in 1963: “There is little doubt that the time is not far when it will be possible to produce all known amino acids with the help of microorganisms.” This time had already arrived in the 70s. Superproducing microbes from the genera Brevibacterium, Corynebacterium, Micrococcus and others were obtained, with the help of which large-scale production of not only glutamate, but also L - lysine, L - valine, L - histidine and others was mastered. During superproduction, the level of expression of the cloned gene is expressed in the synthesis of a specific protein in the amount of 2% of all soluble proteins of the host cell. Currently, there are producers in which the amount of synthesized specific protein reaches 10-15% (multi-copy plasmids that carry built-in genes play the most important role here). Using genetic engineering methods at the All-Russian Research Institute of Genetics and Selection of Industrial Microorganisms (Moscow), an Escherichia coli strain was obtained with overproduction of L-threonine (30 g/l for 40 hours of fermentation).
Any strain that produces any amino acid requires careful and careful handling in order to maintain it in an active state for a long time.
An Escherichiacoli strain was obtained that produced 27 g/l L - proline in 48 hours, and a strain that produced up to 22.4 g / l L - phenylanine.
Using Corynebacteriumsp. can be obtained in alkali-containing media L - tyrosine (up to 19 g/l); using Corynebacteriumglutamicum on glucose medium - L - valine (up to 11 g / l; L - arginine, L - histidine, L - isoleucine - 15 - 20.8 g / l.
3. BIOSYNTHESIS OF AMINO ACIDS
The technology for producing amino acids is based on the principles of fermentation of producers and the isolation of secondary metabolites, that is, the mother culture is first propagated on an agar medium in test tubes, then on a liquid medium in flasks, inoculators and seeding devices, and then in the main (main) fermenters. The processing of culture liquids and the isolation of amino acids is carried out according to a scheme similar to the scheme for obtaining antibiotics. Isolated pure crystals of the desired product are usually dried under vacuum and packaged.
3.1 One-step method for obtaining amino acids
There are two known methods for obtaining amino acids: one-step and two-step. According to the first method, for example, a mutant polyauxotrophic amino acid-producing strain is cultivated on a medium optimal for biosynthesis. The target product accumulates in the culture fluid, from which it is isolated according to the scheme in Figure d
1 - fermenter,
2 - cooler, 3.9 - refrigerators,
4 - container for pre-treatment,
5 - centrifuge,
6 - vacuum - evaporator,
7 - straight apparatus
8 - drum filter, A, B - paths (closing if necessary),
10 - apparatus for ultrafilation,
11 - container for preserving the enzyme solution,
12 - membrane filter,
13 - storage tank for liquid preservative, 14 - container for enzyme precipitation,
15 - filter - press,
16 - spray dryer,
17 - dry concentrate storage.
Figure No. 1 Approximate technological scheme for obtaining amino acids.
3.2 Two-step method for obtaining amino acids
In a two-stage method, the producing microbe is cultivated in a medium where it is produced and synthesizes all the necessary ingredients for the subsequent synthesis (in the idiophase) of the target product.
If amino acid biosynthesis enzymes accumulate intracellularly, but after the 1st stage the cells are separated, disintegrated and used cell sap. In other cases, cells are used directly for the biosynthesis of target products.
3.3 Obtaining lysine
If an amino acid is provided as an additive to feed, then the biotechnological process of the feed product includes the following stages: fermentation, stabilization of the amino acid in the culture liquid before evaporation, vacuum evaporation, standardization of the evaporated solution when adding filler, drying and packaging of the finished product, which should contain more than 10% of the main substance. For example, dry feed and liquid feed concentrates of lysine are produced in industry along with crystalline lysine. (Fig. 2)
Figure No. 2
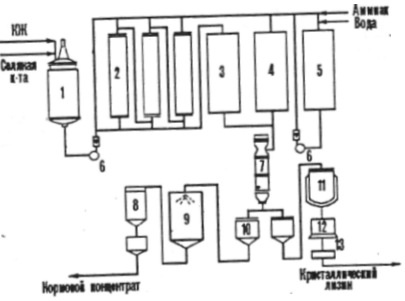
1 - container for culture fluid (CL),
2 - ion exchange columns,
3 - collection of zluat,
4 - filtrate collector,
5 - container for eluate,
7 - vacuum - evaporator,
8 - cyclone,
9 - feed concentrate dryer,
10 - collection,
11 - reactor - crystallizer,
12 - centrifuge,
13 - dryer.
If the concentrate contains 70 - 80% dry matter, then it is quite resistant to microbial spoilage due to the increased osmotic concentration of the ingredients.
3.4 Production of amino acids using immobilized enzymes and cells
Methods for producing amino acids using immobilized enzymes and cells are economically feasible. The process of obtaining L - aspartic acid from fumaric and ammonia in one stage using immobilized E. coli or Pseudomonasaeruginosa cells, which has aspartase activity, was implemented relatively long ago (see diagram)
Aspartase catalyzes the reaction of ammonia adding to fumaric acid. The enzyme in an immobilized state retains activity at the initial level for 2 -2.5 weeks or more.
L - Aspartic acid can also be obtained using immobilized cells, which significantly increases the duration of operation of the system, the productivity of which for the target product is about 2000 kg per 1 m reactor. Batch fermentations are used to produce other L - amino acids (glutamic, phenylalanine, lysine, tryptophan, etc.). In this case, special mutant strains are usually cultivated, the metabolism of which for the target product has been studied quite fully. For example, it has been established that the limiting agent of Corynebac germium, which forms glutamic acid, is the biotype at a dose of 1 - 5 μg/l. Biotin induces structural and functional changes in cell membrane, due to which its permeability to glutamic acid leaving the cell into the culture liquid increases. Some producer strains are capable of accumulating more than 50 g/l on molasses media.
The role of biotin is similar in the case of proline, which is a derivative of glutamic acid.
The simplicity of this technology and its advantages over deep fermentation are clearly illustrated by the experience of the Japanese company Tanabe Seiyaku. In 1973, this company developed a method for producing aspartic acid using immobilized bacterial cells with aspartase activity. Aspartase catalyzes the addition of ammonia at the fumaric acid double bond, i.e. aspartic acid is formed in one stage and this biotechnological process can be classified as biotransformation organic compounds. The enzyme immobilized in the gel functioned well; its half-inactivation period was 1 month. Then the producer cells were immobilized in the gel, further stabilizing them by chemical binding to each other and to the gel. The duration of cell semi-inactivation in this case increased to 4 months. The technology of biotransformation of fumaric acid can thus be presented in the following sequence:
growing cells using deep fermentation and isolating them by centrifugation;
immobilization of biocatalyst cells in a gel in the form of granules 2-3 mm in size;
biotransformation of ammonium fumarate in a column with a catalyst in flow mode and obtaining a solution of aspartic acid;
crystallization, centrifugation and washing of crystals.
The capacity of the aspartic acid biotransformation system for 1 m of bioreactor is 1700 kg.
3.5. Technology for producing glutamate.
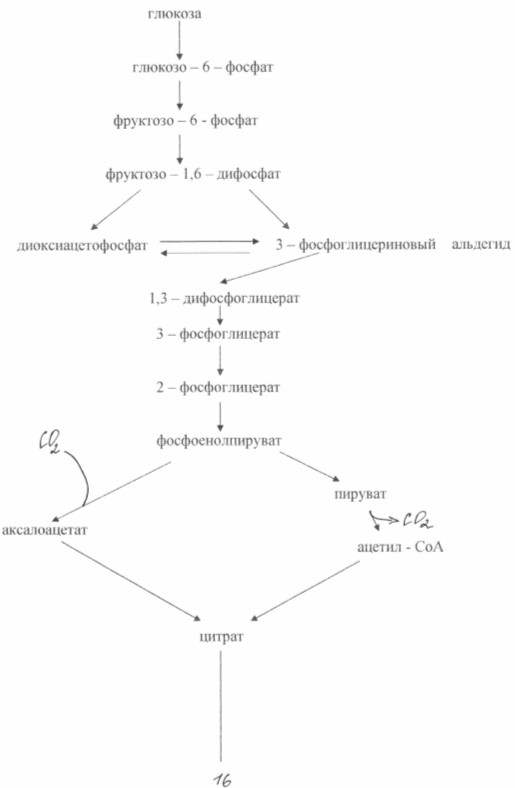
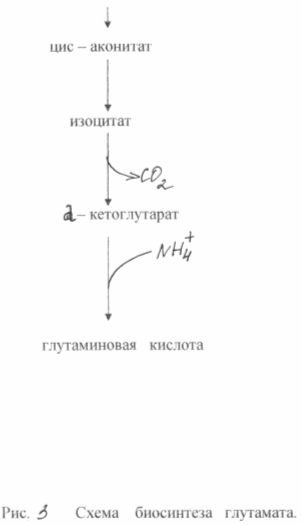
The oversynthesis of glutamic acid from glucose in these bacteria is based on two biochemical principles: a lack of the enzyme a - ketoglutarate dehydrogenase and blocking the biosynthesis of biotin. The inability of cells to synthesize biotin leads to an increase in the permeability of the cytoplasmic membrane, which increases the excretion of glutamate. It is formed as a result of amination of α-ketoglutarate, which is incapable of further transformations in the tricarboxylic acid cycle. Scheme of glutamate biosynthesis from glucose in of this type mutants is shown in Fig. 3
During the biosynthesis of glutamic acid, very great importance has the concentration of biotin in the medium. It is necessary to ensure its concentration is 1 - 5 µg/l. In this case, the normal synthesis of membrane phospholipids is disrupted and the latter becomes permeable to glutamate. At a biotin concentration of 15 μg/l, intensive growth of biomass is observed. The permeability of the cytoplasmic membrane to glutamate can also be reduced by adding penicillin to the medium during the logarithmic phase of growth. In this case, phospholipids are extracted from the membrane and glutamate transport can occur within 40 - 50 hours. Bacterial synthesis of glutamate makes it possible to obtain approximately 50% yield of the product from sugar and accumulate up to 200 g/l of glutamate in the fermentation medium. There are known methods for producing glutamate using ethanol media (up to 60 g/l) or acetate (up to 98 g/l).
4. INDUSTRIAL SYNTHESIS OF AMINO ACIDS
The industrial production of amino acids is carried out in two ways: microbiological and chemical.
4.1 Microbiological synthesis
Microbiological synthesis is based on growing certain types of microorganisms on nutrient media having a suitable carbon source. Most often these are sugars contained, for example, in molasses. Mutated microorganisms with impaired nitrogen metabolism release a large amount of any one amino acid into the solution. After the fermentation process is complete, the amino acid is isolated from the solution using chemical methods
By microbiological fermentation, the main amount of glutamic acid and all lysine are obtained. This process has its advantages and disadvantages. On the one hand, it has few stages and requires relatively simple and versatile equipment. On the other hand, living microorganisms with which one has to work are very sensitive to the slightest change in conditions, and the concentration of the target product is low, which leads to an increase in the size of the equipment.
There is a method for microbiological production of phenylalanine using a tyrosine- and methionine-deficient mutant of Brevibacterium lactofermentum. In the batch fermentation process, a product concentration of 24.8 g/l was achieved. However for this process complex and expensive environments are required. Of particular interest is the biosynthesis of phenylalanine by an auxotrophic mutant of E. coli, which can be cultivated in a glucose medium with phosphates. The fermentation process is carried out using the top-up method with biomass recycling. The biomass in the reactor at the 60th hour reaches 45 - 50 g/l, and the concentration of phenylalanine is 22.4 - 22.8 g/l. System productivity 0.72-0.86 g/(lh); product yield 0.11 g.
4.2 Chemical synthesis
Chemical synthesis is more universal than microbiological synthesis and allows one to obtain compounds of any possible structure. Here, non-food mineral raw materials are used, any concentration of the product is achieved, however, as a rule, the process is multi-stage and requires more complex equipment.
Both methods ensure the production of natural amino acids of the required degree of chemical and optical purity. So ultimately, when it comes to industrial production, the last word remains with the economy: according to foreign experts, on a large scale chemical methods become more profitable.
The most widely developed industrial synthesis is methionine, an amino acid whose main consumer is poultry farming. The starting material is propylene, a product of petroleum cracking. Propylene is oxidized to acrolein, which, through a series of reactions, is converted to racemic methionine.

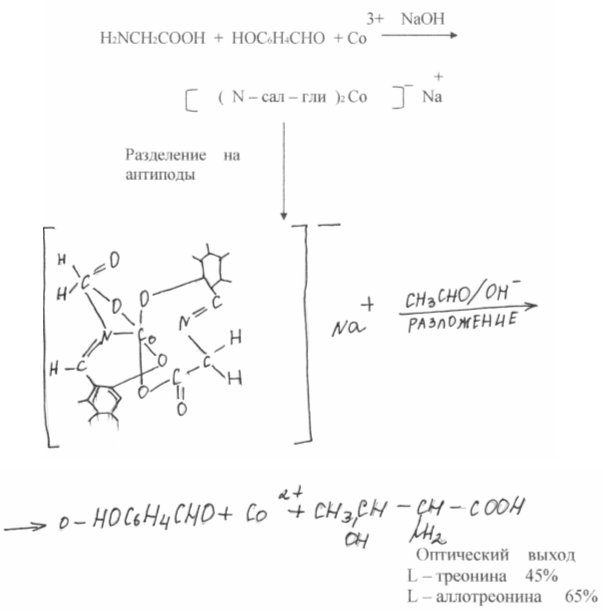
As a result of chemical synthesis, a mixture of equal amounts of L and D isomers of amino acids is usually obtained, while proteins contain exclusively L isomers. These same isomers are nutritious. D-isomers, as a rule, are not absorbed by the body and are ballast. Consequently, separation is necessary, which inevitably has a negative impact on the economy. Recently, significant progress has been made in the degradation of racemic mixtures of amino acids. In the works of SV. Rogozhin and V.A. Davankov showed that optically inactive amino acids, being covalently attached to an insoluble polymer carrier, easily form complexes with copper, nickel, etc. another racemic amino acid in solution occupies two vacant coordination sites at the metal atom, and the strength of the complexes of the L and D isomers is different. No matter how small this difference, when repeated many times during the chromatography process, it provides complete or partial separation of optical antipodes. The best results are obtained with DL - proline, which can be preparatively separated into optical isomers.
The efforts of many researchers are also aimed at developing a chemical synthesis that would give only one desired natural optically active isomer - the isomer synthesized by living nature - asymmetric synthesis. In this direction for last years serious progress has been made. In the works of A. Kagan (France) and E. Corn (USA), almost quantitative optical outputs were achieved. It is extremely tempting to reproduce the pathways of amino acid synthesis using natural enzyme systems. A large number of Such syntheses are carried out by pyroxal-dependent enzymes, and the desired optical isomer of the amino acid is immediately obtained. A great contribution to the study of these processes was made by Academician A.E. Braunstein (Russia) and Professor Yu. Snell (USA).
Russian scientists have set themselves the goal of finding chemical systems that could not only simulate biochemical reactions, but also carry out processes that do not take place in a living organism. Complexes of Schiff bases of amino acids with transition metal ions were chosen as such a system. It was assumed that salicylic aldehyde in these complexes would play the role of lyridoxal, increasing the reactivity of the C-H bond of the amino acid; the metal ion would do the same, but still keep the system in a rigid flat state, which in nature provides the enzyme protein. The general dissymmetry of the complex allowed us to hope that it is provided by an enzyme protein in nature. The general dissymmetry of the complex allowed us to hope that the reaction could be carried out stereospecifically, i.e. result in an optically active acid.
Such reactions do not occur in nature, but their practical value lies in the fact that glutamic acid is immediately obtained. Thus, the path opens for a new general synthesis of amino acids, taking place under extreme conditions.
It will be incomparably more difficult to reproduce the other side of the action of natural enzymes - asymmetric synthesis. For this purpose, the complexes were divided into optically active antipodes.

5. APPLICATION OF AMINO ACIDS
The proteins of all organisms, from viruses to humans, consist of 21 amino acids, which, according to their biological value, are divided into replaceable (the body can synthesize them itself at a sufficient speed to build proteins) and essential (it cannot synthesize them and must obtain them from outside food). Each protein contains a certain amount of each amino acid. If there is no or little essential amino acid in the protein consumed, the body's protein will not be built. Hence the need to balance the diet, which leads to an increase in their nutritional value.
In Figure (4), casein, a nutritious animal protein, is taken as ethanol. The shaded portion of each column represents the nutritional value of the natural protein in PER (Protein Efficiency Ratio) units. Adding a certain amount of lysine, the first limiting amino acid, to the product leads to a sharp increase in nutritional value; adding a second limiting amino acid increases nutritional value to the level of animal proteins.
Rice. 4 Increasing the nutritional value of protein by adding limiting amino acids in EBC units
Diet balancing is widely used in agriculture. According to M.F. Tomme, I.F. Tkachev, the inclusion of 0.2 - 0.5% lysine in the diet of piglets and chickens can reduce feed protein consumption by 25% and increase animal productivity by 10-13%. Summarizing the results of research by both Russian and foreign authors, we find that by organizing the production of 20 thousand tons of lysine per year, you can obtain an additional 1.2 million tons of meat and save 3.6 million tons of protein feed. According to the Dutch economist N. Marguder, 0.125% lysine in pig feed consisting of cornmeal and groundnuts gives a net profit of 100%.
Balancing grain products with pure amino acids has not yet found wide application in the food industry, although it improves the nutritional properties of plant protein, and therefore increases the amount of protein suitable for use in food. The latter is especially important, since there is a real opportunity to meet the rapidly growing needs for dietary protein without simultaneous changes in the nature of diets and the organoleptic properties of products. Amino acids are no less successfully used in medicine. After severe surgery, burns, etc. The human body very often cannot absorb protein in the amount necessary for regeneration processes, and this leads to a deterioration in the patient’s condition. Then they resort to amino acid nutrition. The excellent therapeutic effect achieved in these cases causes a great need for amino acid mixtures. A mixture that does not contain phenylalanine is fed to children suffering from phenylpyruvic oligophrenia.
Amino acids serve as starting materials for the synthesis of polypeptides, many of which are the strongest physiologically active compounds, as well as for the creation of other drugs.
Finally, in the process of polycondensation, amino acids form polymers, which, while not possessing the biological activity of proteins, are similar to them in their structure and limit some of their important physical properties. Artificial wool, silk and leather obtained with the participation of amino acids are not inferior to natural materials in performance indicators.
Despite the widespread use of amino acids in medicine, industry and agriculture, their main consumer remains the food industry. Thus, monosodium glutamate (sodium salt of glutamic acid) is a powerful flavor enhancer. In many countries it is added to all products during canning, freezing, and long-term storage; it is used both in public catering and in the household in the form of a mixture with table salt. A mixture of amino acids is also used to create compositions that imitate the taste and smell of food products.
Consumption of amino acids in the world increases annually by 10%. Their production is characterized by the following figures
Conclusion
This course work dedicated to the industrial biosynthesis of amino acids. Thanks to this work, I found out that there are the following ways to obtain amino acids:
1. Biosynthesis of amino acids, which includes one-step and two-step methods;
2. Industrial synthesis of amino acids - microbiological and chemical synthesis.
We considered the production of amino acids using immobilized enzymes and cells, as well as the technology for producing lysine and glutamate.
She established the use of amino acids not only in medicine, but also in agriculture in order to reduce the consumption of feed protein; and in the food industry as preservatives and flavor enhancers
List of sources used
1. E.A. Stroev. Biological chemistry. M., graduate School, 1986.
2. M.E. Becker, G.K. Liepinin, E.P. Raipulis. Biotechnology. M., VO Agropromizdat, 1990.
3. U.E. Viestur, I.A. Shmite, A.V. Zhilevich. Biotechnology. Biotechnological agents, technology, equipment. Riga, Zinatne, 1987.
4. G.K. Liepinin, M.E. Dongze. Raw materials and nutrient substrates for industrial biotechnology. Riga, Zinatne, 1986.
5. N.P. Blinov. Fundamentals of biotechnology. St. Petersburg, Nauka, 1995.
6. L.I. Vorobiev. Industrial microbiology. M., Moscow State University, 1989.
7. S.D. Varfolomeev, S.V. Kalyuzhny. Biotechnology. Kinetic Basics microbiological processes. M., Higher School, 1990.
8. V.M. Belikov. Amino acids, their chemical synthesis and application. Vestn. USSR Academy of Sciences, 1973.
9. J. Bailey, D. Ollis. Fundamentals of biochemical engineering, vol. 1. M„ Mir, 1989.
10. G.S. Murovtsev, R.G. Butenko, T.N. Tikhonenko, M.I. Prokofiev. Fundamentals of agricultural biotechnology. M., VO Agropromizdat, 1990.
11. Biotechnology: principles and applications. Ed. I. Higgins, D. Best, J. Jones. M., Mir, 1988.
Practical significance of the topic. Amino acids that enter the body with food can be sources of carbon and nitrogen atoms for various non-protein compounds. In particular, amino acids can enter into interconversion reactions, which ensures the body’s needs for non-essential amino acids, regardless of their supply from the outside. Physiologically important products formed from amino acids include heme, creatine, choline, purine and pyrimidine nucleotides, some hormones, and neurotransmitters. Knowledge of these metabolic pathways is important for understanding the pathogenesis of some hypovitaminosis and congenital enzymopathies, and developing methods for their biochemical diagnosis and correction.
Purpose of the lesson. After studying this topic, the student should know the basic reactions of the synthesis of non-essential amino acids in the human body, the ways of using amino acids for the synthesis of non-protein nitrogenous compounds and possible violations of these processes, and be able to apply the acquired knowledge to solve theoretical and practical problems.
Initial level of knowledge.
The structure of amino acids (glycine, serine, methionine, cysteine, phenylalanine, tyrosine).
Reactions of transfer of functional groups in biological systems.
Reductive amination of α-keto acids: enzymes, role in the body.
Transamination of amino acids: reactions, role in the body.
Coenzyme functions of vitamins (folic acid, cyanocobalamin).
Principles for diagnosing congenital enzyme defects.
3.1. Biosynthesis of amino acids in tissues.
3.1.1. The human body can synthesize non-essential amino acids, which include: alanine, arginine, aspartate, histidine, glycine, glutamate, glutamine, proline, serine, tyrosine, cysteine. A dietary deficiency of any of these amino acids will not be accompanied by a deficiency in the body. The main ways of formation of non-essential amino acids are: 1) transamination of α-keto acids, 2) reductive amination of α-keto acids, 3) synthesis involving essential amino acids.
3.1.2. Transamination(see topic 2). The sources of carbon atoms in these reactions are metabolites of glycolysis and the Krebs cycle, and the sources of nitrogen atoms are other amino acids, most often glutamate (see Figure 7).
3.1.3. Reductive amination(see topic 2). The source of the nitrogen atom of the amino group is an ammonia molecule, the source of carbon is α-keto acids, most often α-ketoglutarate (see Figure 3.1).
Figure 3.1. Biosynthesis of non-essential amino acids in tissues using the carbon skeleton of glucose (one asterisk indicates transamination reactions, two - reductive amination).
3.1.4. Synthesis involving essential amino acids. The nonessential amino acid tyrosine can be formed from the essential amino acid phenylalanine:
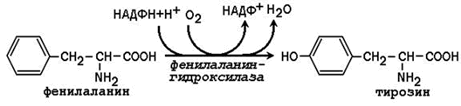
Phenylalanine hydroxylase is a typical cytochrome P 450-dependent hydroxylase with a mixed function: one oxygen atom is included in water and another in the hydroxyl group of tyrosine. The reducing agent is the cofactor tetrahydrobiopterin, which is maintained in a reduced state by the NADPH-dependent enzyme dihydrobiopterin reductase.
The nonessential amino acid cysteine is synthesized with the participation of the essential amino acid methionine, which is used as a source of sulfur atom. After donating a methyl group in transmethylation reactions, methionine is converted to homocysteine. When it interacts with the non-essential amino acid serine, cystathionine is formed:
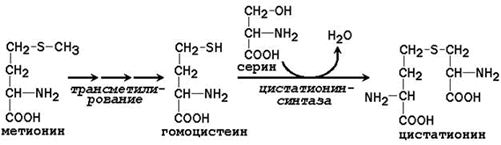
Cystathionine undergoes cleavage to form cysteine and homoserine, which undergoes deamination to α-ketobutyrate:
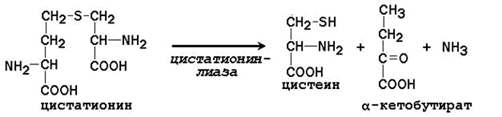
Thus, phenylalanine and methionine supplied from food are partially used for the synthesis of non-essential amino acids. Therefore, the daily requirement for phenylalanine and methionine can be significantly reduced by supplementing the body with additional amounts of tyrosine and cysteine, respectively.
