In the course of studying the topic “Fundamentals of molecular kinetic theory,” we examined the so-called isoprocesses, that is, processes that occur at a constant one of the macroscopic parameters (pressure, volume or temperature). When substituting these data into the Clapeyron equation, these conditions slightly modified it. Now we will do a similar analysis, but with the first law of thermodynamics:
Let us consider the case of the process occurring at a constant temperature:
Hence:
![]()
This means that the first law of thermodynamics takes the form:
Having considered the case of positive work being performed on a gas by external forces:
That is, during isothermal compression or expansion, the gas releases heat into the environment.
Let's consider the case when the process occurs at constant pressure:
Since no factor is included in any thermodynamic quantity, no term of the first law of thermodynamics is reset, and it retains its previous form:
Now let's consider what changes will occur in the recording of the first law of thermodynamics if the process occurs in a fixed volume.
Hence:
![]()
This means that the first law of thermodynamics takes the form:
That is, the meaning of using isochoric processes in heat engines is lost, because all the energy of the burned fuel will be used to change internal energy, and the gas will not do any useful work.
There is also another very important process- adiabatic.
Definition.Adiabatic process - a process occurring in a thermally insulated system. That is, without supplying gas or releasing heat from gas.
The first law of thermodynamics takes the form:
That is, as a result of work being done on a gas by external forces, its internal energy, and therefore the temperature, increases, and when work is done by the gas, it decreases.
The following experiments are clear examples of the last two statements.
1. A cylinder closed by a moving piston contains a small amount of fuel. After quickly pressing the piston, the fuel ignites.
2. In a container closed with a stopper and threaded with a pump hose, there is a small amount of water. After a certain amount of air is pumped into the vessel, the stopper quickly flies out and fog is observed in the vessel (Fig. 1).
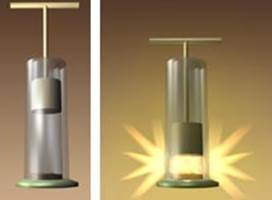

Rice. 1. Illustrative examples adiabatic processes ()
In two cases, the gas performs work of different signs, and, therefore, the change in internal energy and temperature has different signs. In both cases, the emphasis is on a rapid change in volume because it is impossible to create a perfectly isolated system, but if we consider a very transient process, then the heat will not have time to be transferred, and the process can be considered adiabatic.
Graph of adiabatic processes in P-V coordinates looks like this (Fig. 2).
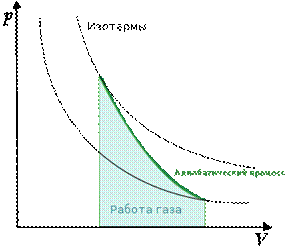
Rice. 2. Graph of the adiabatic process ()
Starting from the next lesson, we move on to studying a new section of physics - electrostatics.
Bibliography
- Myakishev G.Ya., Sinyakov A.Z. Molecular physics. Thermodynamics. - M.: Bustard, 2010.
- Gendenshtein L.E., Dick Yu.I. Physics 10th grade. - M.: Ilexa, 2005.
- Kasyanov V.A. Physics 10th grade. - M.: Bustard, 2010.
- Flash-fizika.narod.ru ().
- Youtube.com().
- Nashaucheba.ru ().
Homework
- Page 82: No. 635-639. Physics. Problem book. 10-11 grades. Rymkevich A.P. - M.: Bustard, 2013. ()
- With the adiabatic expansion of 64 g of oxygen under normal conditions, the temperature increased by 2 times. How much work has been done by the gas?
- Determine the amount of heat imparted to 2 kg of helium at a constant volume if its temperature increased by 100 K. How much did the internal energy of the gas change and how much work was done by it?
- * Why, if you knock off the valve of a compressed air cylinder, frost appears on the neck of the cylinder?
According to the first law of thermodynamicsΔ U =Q+A.
Isothermal, isochoric and isobaric isoprocesses are widely used in technology. So, Gay-Lussac's law forms the basis for the structure of gas thermometers; Charles's law“works” in devices called autoclaves, etc. Thermodynamics studies another process that is widely used in practice, in particular in heat engines. This is the so-called adiabatic process.
Adiabatic process is a thermodynamic process that occurs in a thermally insulated system, that is, in the absence of heat exchange with surrounding bodies.
Because in this case Q = 0 , then, in accordance with the first law of thermodynamics, all the work performed goes to change the internal energy of the system: A=Δ U.
Of course, in real conditions it is almost impossible to achieve such a result, since there are no ideal heat insulators. But there are several ways to approach this condition. For example, to create shells with low thermal conductivity (according to the principle of a thermos) or to carry out the process so quickly that the heat exchange between the system and the surrounding bodies would be short-lived and could be neglected.
At adiabatic compression Ga-for all the work performed goes to increase the internal energy of the body:A = Δ U.At adiabatic expansion gasA'= — Δ U,that is, the gas does work by reducing its own internal energy.
For example, rapid compression of a gas causes an increase in internal energy, which is equal to the amount of work done A, and the gas heats up. This phenomenon, in particular, is the basis for the spontaneous combustion of the fuel mixture in diesel engines. And vice versa, if the gas itself performs work due to rapid expansion, then its internal energy decreases and the temperature of the gas decreases. This property of the adiabatic process is the basis for gas liquefaction. An example of an adiabatic process is also an explosion, melting of a fuse during a short circuit, etc.
Adiabats, like isotherms, do not intersect each other.Material from the site
Graphically on the coordinate plane pV the adiabatic process is depicted by a curve called adiabatic(Fig. 2.5). It falls steeper than the isotherm, since in an adiabatic process the change in pressure occurs due to a simultaneous increase in volume and a decrease in temperature. This conclusion is also confirmed by formula (24): p =nkT, after all, an increase in gas volume leads to a decrease in the concentration of gas molecules, and therefore a decrease in pressure is determined by two parameters - gas temperature T and concentration of molecules n.
Due to adiabatic expansion gas there is a change in its state, which is characterized by a decrease in internal energy; at adiabatic compression gas, its internal energy increases.
On this page there is material on the following topics:
Isoprocesses formulas cheat sheet
Physical chemistry adiabatic process
Physics adiabatic process
Adiabatic process physics cheat sheet
Adiabatic process report
Questions about this material:
An adiabatic process is a process without heat exchange with the external environment. In an adiabatic process, energy exchange between the working fluid and the environment occurs only in the form of work; there is no energy exchange in the form of heat. These conditions are expressed by the relation: . Then the equation of the first law of thermodynamics for an adiabatic process has the form:
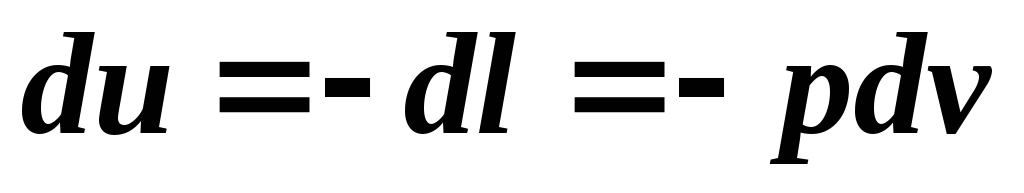 .
(5.4)
.
(5.4)
From this equation it is clear that the work of the adiabatic expansion process is performed due to a decrease in the internal energy of the gas and, consequently, the temperature of the gas decreases. The work of adiabatic compression goes entirely to increasing the internal energy of the gas, that is, increasing its temperature. Thus, the change in internal energy and work in an adiabatic process are equivalent in magnitude and opposite in sign.
Let us derive the adiabatic equation for an ideal gas. Let's use the equation of the first law of thermodynamics:
Dividing the variables, we get:
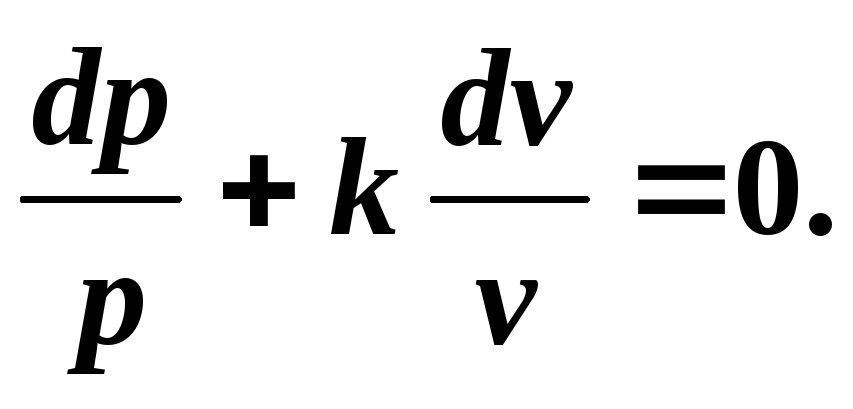 (5.6)
(5.6)
Integrating (5.6) at k = const , we get where
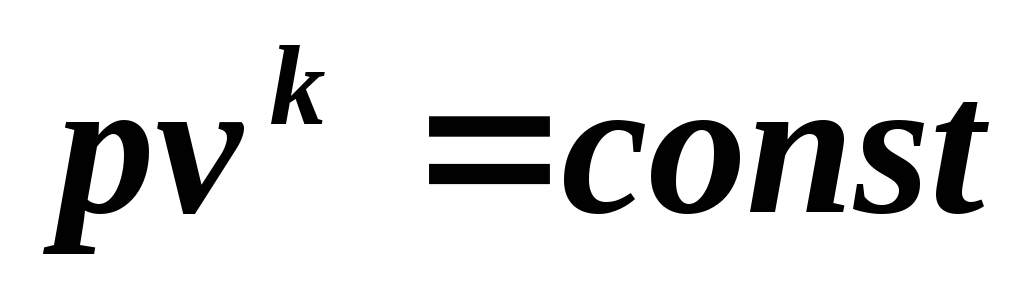 (5.7)
(5.7)
Equation (5.7) is an adiabatic equation.
In Fig. Figure 5.10 shows the adiabatic process of gas expansion in  -diagram.
-diagram.
From equation (5.7) it follows:
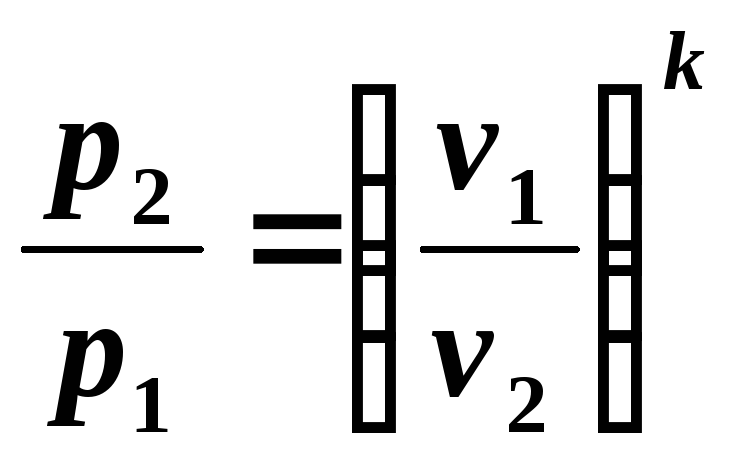 , (5.8)
, (5.8)
that is, during adiabatic expansion the pressure drops, and during compression it increases.
Rice. 5.10. Adiabat of an ideal gas
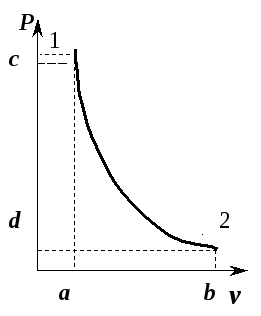
Considering that in an adiabatic process all three state parameters change, it is necessary to identify the relationships between v And T , p And T .
Relationship between temperature T and volume v can be obtained from equation (5.8) and equations of state written for process points 1 And 2 : R 1 v 1 = RT 1 And R 2 v 2 = RT 2 , where
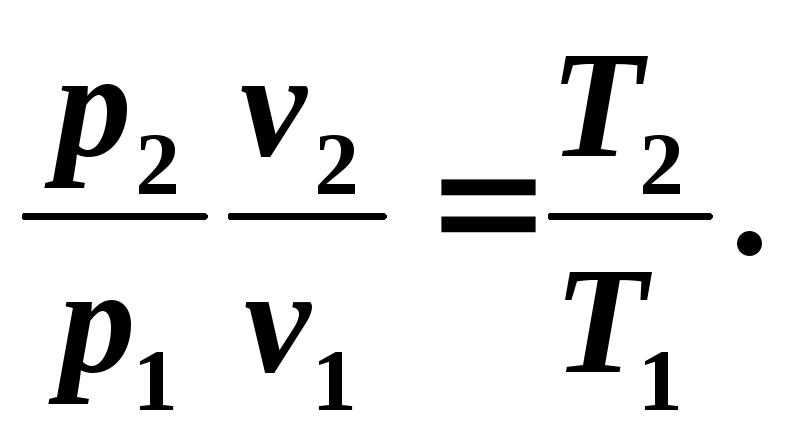 (5.9)
(5.9)
From equations (5.8) and (5.9) it follows: 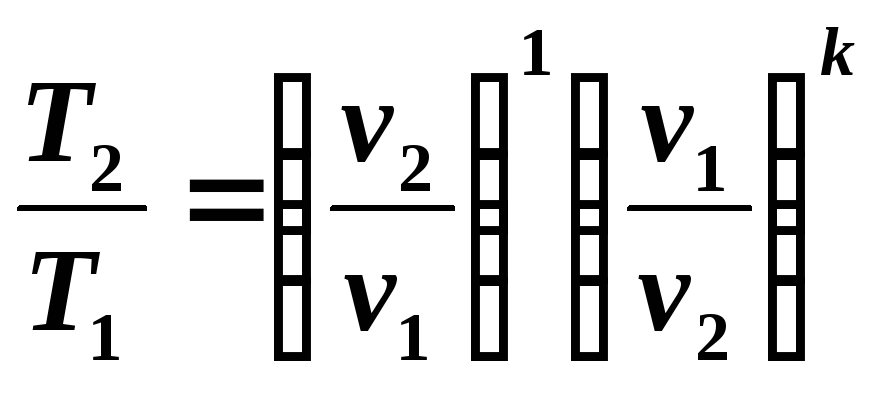
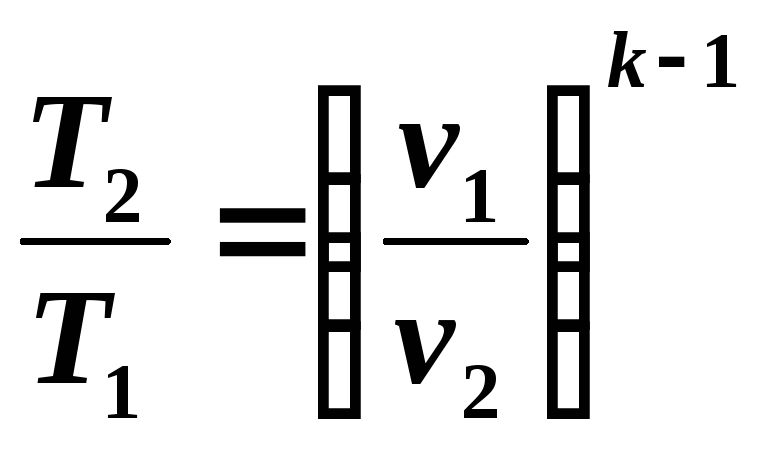 (5.10)
(5.10)
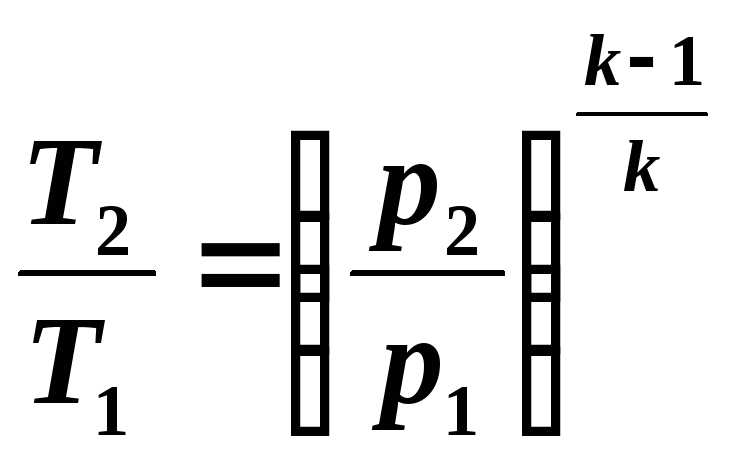 (5.11)
(5.11)
At k
=
const
To calculate the work of an adiabatic process, you can write several formulas. From Eq. 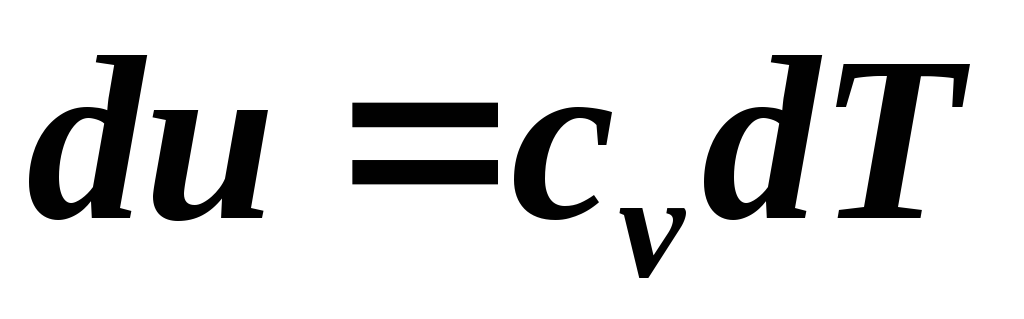 at c
v
=
const
we have:
at c
v
=
const
we have:
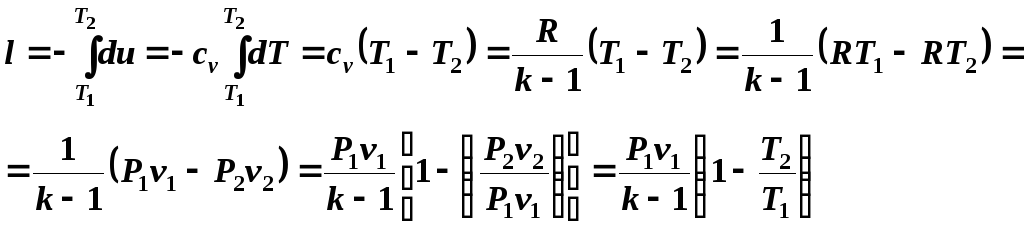 (5.12)
(5.12)
Taking into account relations (5.10) and (5.11), we write equation (5.12) in the form:
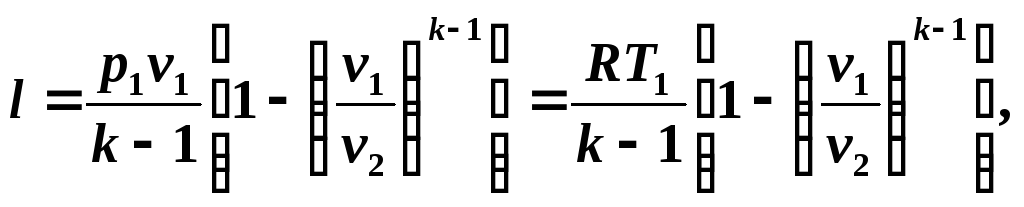
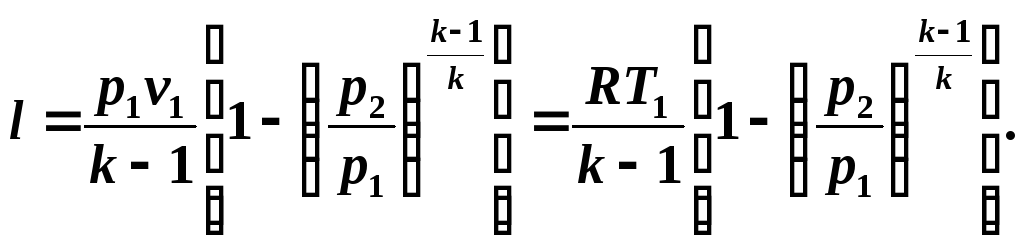
We determine the available work in an adiabatic process from the relation
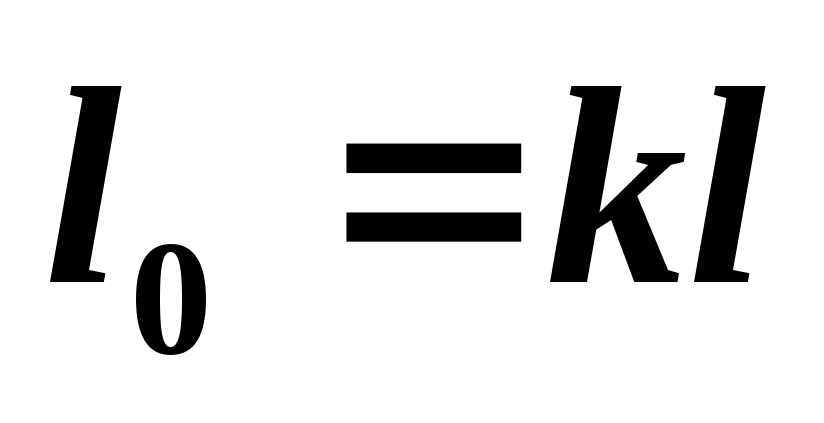 (5.14)
(5.14)
For a reversible adiabatic process 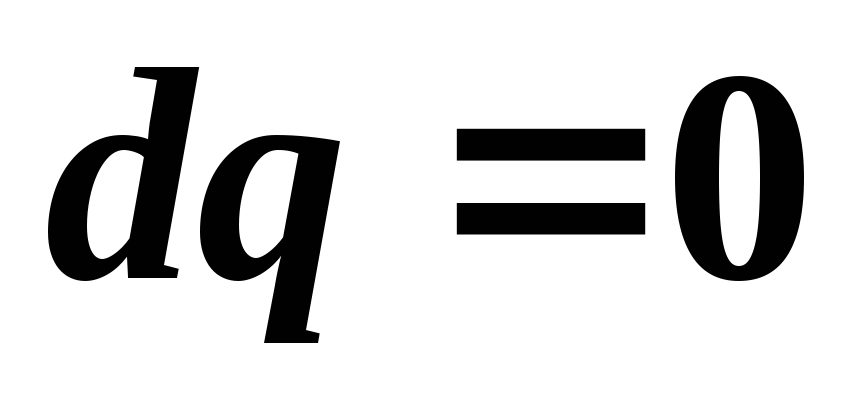 , That's why
, That's why
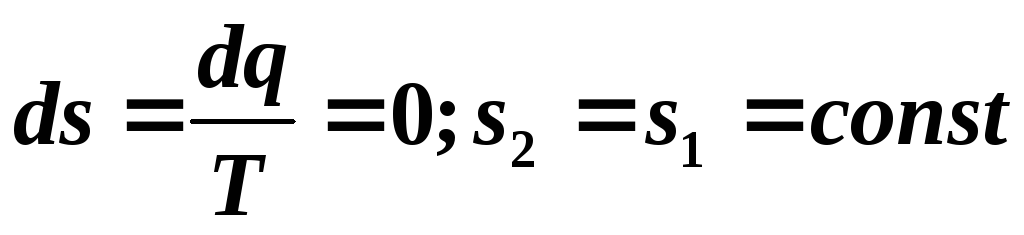 , that is, a reversible adiabatic process will be isentropic and in
, that is, a reversible adiabatic process will be isentropic and in  -the diagram is depicted as a straight line parallel to the axis
-the diagram is depicted as a straight line parallel to the axis  (Fig. 5.11). The process of adiabatic expansion is depicted by a vertical line 2-1 going down, and the process of adiabatic compression 1-2 by a vertical line going up.
(Fig. 5.11). The process of adiabatic expansion is depicted by a vertical line 2-1 going down, and the process of adiabatic compression 1-2 by a vertical line going up.
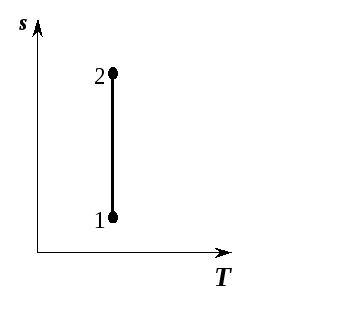
Rice. 5.11. Adiabatic process in  -diagram
-diagram
The heat capacity in an adiabatic process is zero: 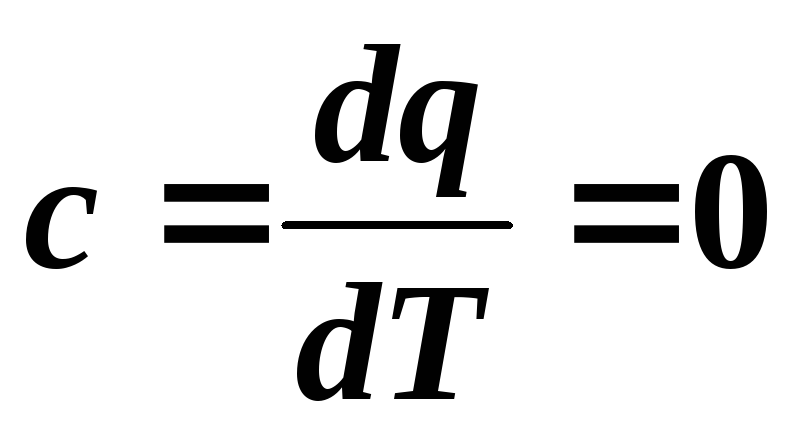 .
.
|
|
|
Rice. 5.12. Scheme of energy distribution in an adiabatic process:
a- during gas expansion; b – during gas compression
Polytropic process
Polytropic process is any arbitrary process of changing the state of the working fluid, occurring at a constant heat capacity With x , that is c = c x = const. The process line is called a polytrope.
From the definition of a polytropic process it follows that the main thermodynamic processes (isochoric, isobaric, isothermal, adiabatic, if they occur at a constant specific heat, are special cases of a polytropic process.
In other words, a polytropic process is characterized by the same fraction of the amount of input heat spent on changing the internal energy of the system.
The equation of a polytropic process can be obtained from the equations of the first law of thermodynamics for an ideal gas:
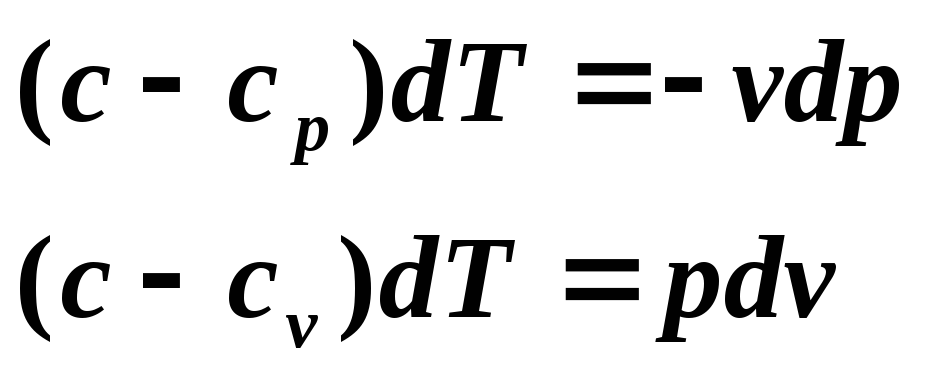
Divide the first equation by the second
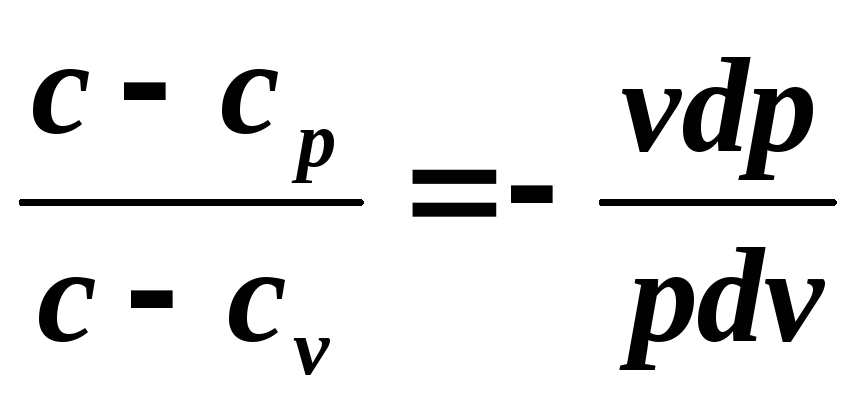
and denote
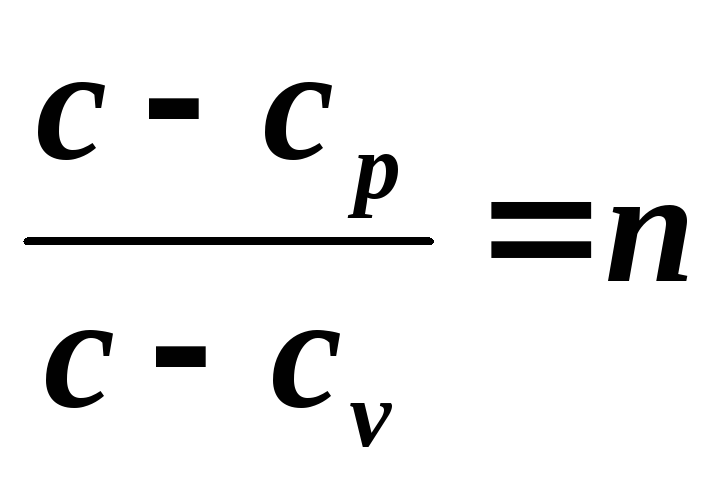 ,
,
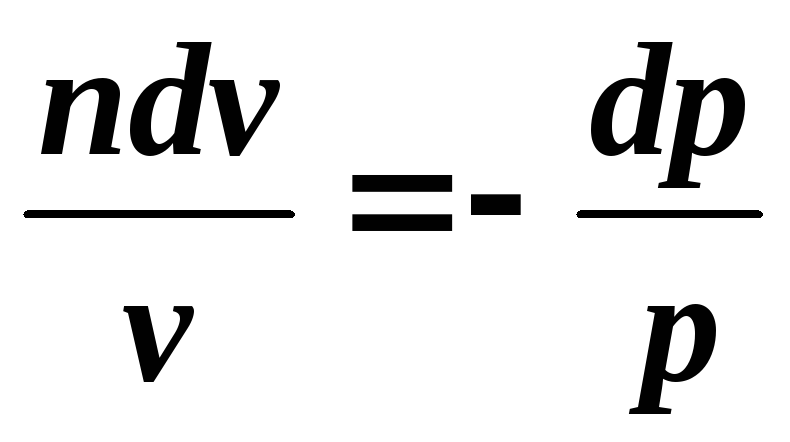
Integrating the resulting relationship from the beginning to the end of the process, we find:
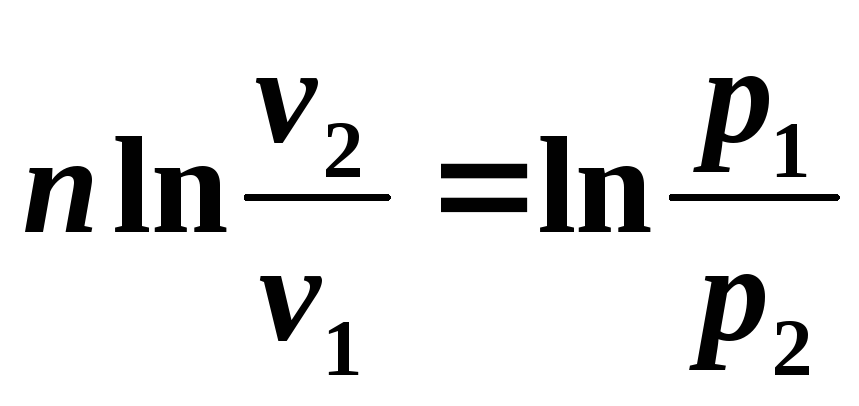 ,
,
or after potentiation
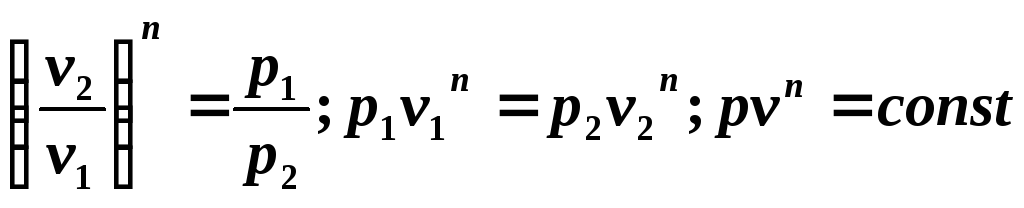
we obtain the equation of the polytropic process 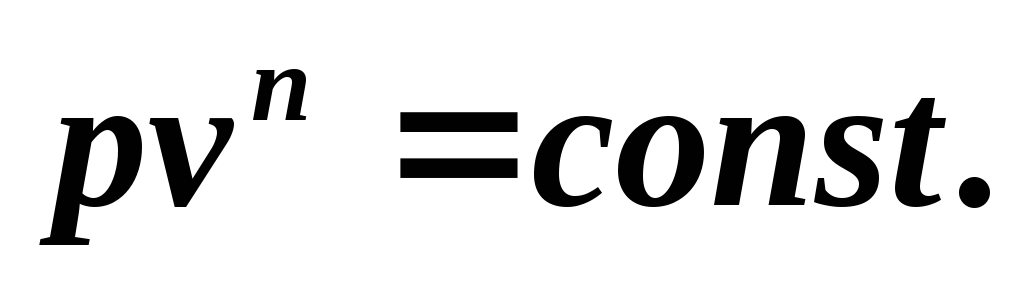
Since the polytropic equation differs from the adiabatic equation only in the value of the exponent n , then all relationships between the main parameters can be represented by formulas similar to the formulas for the adiabatic process:
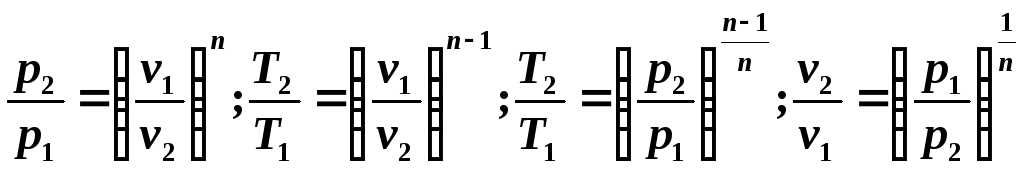
The specific heat capacity of a polytropic process can be determined from the expression for the polytropic index
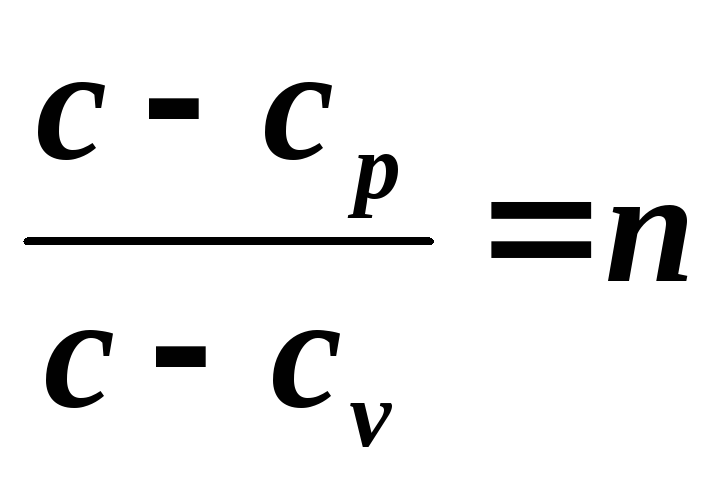 , where
, where 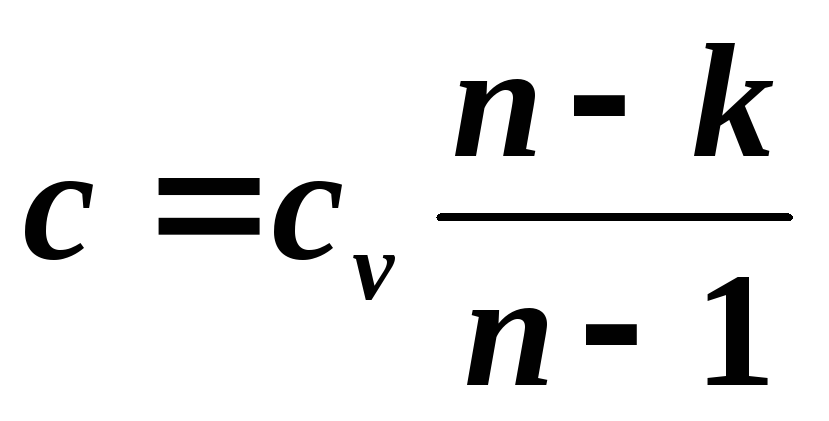 ,
,
Where k – adiabatic index.
The last equation allows us to determine the specific heat capacity of a polytropic process for any value n . If we substitute values for special cases into this equation, we can get:
|
Isochoric process: |
n = ; |
c = c v ; |
v = const. |
|
|
Isobaric process: |
n = 0 ; |
c = kc v =c p ; |
p = const . |
|
|
Isothermal process: |
n= 1 ; |
c = ; |
T = const. |
|
|
Adiabatic process: |
n = k ; | |||
Adiabatic process
Adiabatic is a process in which there is no heat exchange ( = 0) between the physical system and environment. All fast processes are close to adiabatic. For example, the process of sound propagation in a medium can be considered an adiabatic process, since the propagation speed sound wave so great that the exchange of energy between the wave and the medium does not have time to occur. Adiabatic processes are used in internal combustion engines (expansion and compression of the combustible mixture in cylinders), in refrigeration units, etc.
From the first law of thermodynamics () for an adiabatic process it follows that
that is, external work is done due to changes in the internal energy of the system. Thus, adiabatic process is the opposite of isothermal, since in the latter the work is done due to the influx of an equivalent amount of heat from outside.
Using expressions (52.1) and (53.4), for an arbitrary gas mass we rewrite equation (55.1):
![]() . (55.2)
. (55.2)
Differentiating the equation of state for an ideal gas
![]() . (55.3)
. (55.3)
Let us exclude from (55.2) and (55.3) the temperature T:
![]() .
.
Separating the variables and taking into account that (see (53.8)), we obtain
![]()
Integrating this equation over the range p 1 before p 2 and accordingly from V 1 before V 2, and then potentiating, we get
![]() , or .
, or .
Since the states 1 And 2 chosen arbitrarily, we can write
Const. (55.4)
The resulting expression is the equation gas state in an adiabatic process, also called Poisson's equation.
To go to variables T, V or r, T eliminate from (55.4) using the Clapeyron-Mendeleev equation
pressure or volume, respectively:
Const, (55.5)
Const. (55.6)
Expressions (55.4) - (55.6) represent the equations of the adiabatic process. In these equations, the dimensionless quantity (see (53.8) and (53.2))
is Poisson's ratio. For monatomic gases (Ne, He, etc.), which satisfy the ideality condition quite well, = 3, = 1.67. For diatomic gases (H 2, N 2, O 2, etc.) = 5, = 1.4. The values calculated using formula (55.7) are well confirmed by experiment.
Diagram of an adiabatic process ( adiabatic) in coordinates p, V is depicted as a hyperbola (Fig. 83). The figure shows that the adiabat ( = const) is steeper than the isotherm ( pV= const). This is explained by the fact that during adiabatic compression 1-3 An increase in gas pressure is caused not only by a decrease in its volume, as with isothermal compression, but also by an increase in temperature.
Let us calculate the work done by the gas in an adiabatic process. Let us write equation (55.2) in the form
![]()
If a gas expands adiabatically from its volume V 1 before V 2, then its temperature drops from T 1 before T 2 and work of expansion of an ideal gas
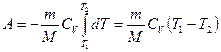 . (55.8)
. (55.8)
Using the same techniques as when deriving formula (55.5), expression (55.8) for work during an adiabatic process can be transformed to the form 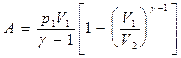
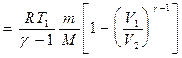
Work done by a gas during adiabatic expansion 1-2 (determined by the shaded area in Fig. 83), less than with isothermal. This is explained by the fact that during adiabatic expansion the gas is cooled, while during isothermal expansion the temperature is maintained constant due to the influx of an equivalent amount of heat from outside.
The considered isochoric, isobaric, isothermal and adiabatic processes have common feature– they occur at constant heat capacity. In the first two processes, the heat capacities are respectively equal C V and C p in an isothermal process (dT=0) the heat capacity is equal to ±∞, in an adiabatic process (δQ=0) the heat capacity is zero. The process in which the heat capacity remains constant is called polytropic.
Based on the first law of thermodynamics, under the condition of constant heat capacity (C=const), we can derive the polytropic equation:
where is the polytropic index. Obviously, for C=0, n=γ, from (55.9) the adiabatic equation is obtained; at С=∞, n=1 – isotherm equation; at C=C p, n=0 – isobar equation, at C=C V, n=±∞ – isochore equation. Thus, all the processes considered are special cases of a polytropic process.
3.8. Application of the first law of thermodynamics
A thermodynamic process in which a system, when transitioning from state 1 to state 2, does not exchange heat with the environment is called adiabatic.
In practice, an adiabatic process can be carried out with rapid expansion (compression) of a gas, when d Q º0. For example, the rapid expansion of gases in the cylinder of an internal combustion engine. In a Diesel engine, air is rapidly compressed adiabatically 15 times or more than in an internal combustion engine. In this case, the air temperature rises to 3000 o C, so when the combustible mixture is injected, it spontaneously ignites.
Whenever shock wave the gas is adiabatically compressed and becomes very hot, because he does not have time to give up the released heat.
When meteorites enter the atmosphere, they melt and evaporate mainly for this reason, and not due to the presence of friction and resistance when moving in atmospheric air.
Adiabatic expansion leads to cooling of the system, which is used in the liquefaction of gases (adiabatic demagnetization of paramagnetic salts allows obtaining temperatures close to absolute zero). Adiabatic processes also include the free expansion of gases (Fig. 3.6), because Q=0, A=0, D U=0, DT=0.
Rice. 3.6
Let us represent the first law of thermodynamics for an adiabatic process in the form
Consequently, during an adiabatic process, the gas does work due to the decrease in its internal energy.
The heat capacity of a substance during an adiabatic process is C = 0 (d Q=0, dT ¹ 0).
Let us find the form of the equation of state of an ideal gas for an adiabatic process.
Let us rewrite equation (3.21) as:
Applying Mayer's equation (3.16), we transform (3.24) to the form
|
WITH R РdV + C v VdP = 0. |
(3.25) |
Equation (3.25) can be represented as
|
|
(3.26) |
Where
|
(3.27) |
called coefficient Poisson
(adiabatic exponent).After integrating (3.26) taking into account (3.27), we obtain
or
|
(3.28) |
Expression (3.28) is called adiabatic equation (the equation Poisson).
Using the Mendeleev-Clapeyron equation (1.9) we rewrite (3.28) as:
![]() .
.
The P-V diagram of the adiabatic process is shown in Fig. 3.7.
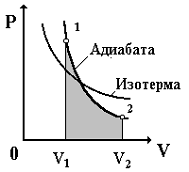
Rice. 3.7
From Fig. 3.7 it is clear that the adiabatic curve is steeper than the isotherm. This is explained by the fact that during the adiabatic expansion of an ideal gas, not only a decrease in pressure occurs, but also a decrease in temperature, because the internal energy of the gas decreases. During adiabatic compression of a gas, pressure and temperature increase, not only due to a decrease in volume, but also due to an increase in internal energy. This can be observed in the computer model "Adiabatic Process".
Computer model "Adiabatic process"
The model is designed to study an adiabatic process, i.e., the process of quasi-static expansion or compression of an ideal gas located in a vessel with heat-tight walls. You can change the initial temperature T of the gas. A graph of the dependence p (V) for an adiabatic process is presented, an energy diagram is displayed, which shows the work A performed by the gas and the change ΔU of its internal energy.