Examination project for the full course high school in the subject "Physics"
GOU Secondary School No. 7
Reutov, Moscow region, 2008
Basic principles of the theory
An image of atoms on a silicon surface obtained using a tunneling microscope.
Starting from the 18th century, a system of scientific ideas about the structure of matter gradually began to emerge, later called the molecular kinetic theory (MKT). The molecular kinetic theory is based on three principles that summarize the results large quantity experimental data:
All bodies consist of tiny particles - atoms, molecules and ions. Thus, any substance has a discrete structure.
The particles that form matter are in continuous chaotic motion, which is called thermal motion.
Atoms, molecules and ions interact with each other.
Let us consider these provisions in some detail.
Molecular structure of matter.
Mole of substance.
Avogadro's number. Amount of substance.
Modern physics does not establish the limits of the structural divisibility of matter, however, it unequivocally states that the elements that determine the basic physical properties of bodies are atoms, molecules and ions.
An atom is the smallest particle of a given chemical element that is the carrier of its properties.
To each chemical element corresponds to its atom.
A molecule is the smallest stable particle of a given substance that has its basic chemical properties.
A molecule consists of atoms of the same or different chemical elements.
An ion is an electrically charged particle that is formed when atoms and molecules lose or gain one or more electrons.
Inert gases (helium, argon, etc.), liquids (mercury) and solids (copper, diamond) have an atomic structure. A number of crystalline substances, for example, sodium chloride, consist of oppositely charged ions. However, the vast majority of substances are formed from molecules ( carbon dioxide, water), therefore the concept of “molecule” is often used as a general collective term.
Modern experimental technology makes it possible to observe the molecular structure of a substance, as well as determine the sizes of atoms and molecules. These sizes are very small: for atoms they are on the order of 10–10 m, for molecules their range is much wider - from 10–10 m for the simplest molecules to 10–5 m for molecules of complex organic substances.
Naturally, with such dimensions, the mass of atoms and molecules is also very small; for example, the mass of a hydrogen molecule is 3.3·10–27 kg. It is clear that it is not entirely convenient to operate with such quantities in practical calculations.
Therefore, the concept of relative molecular (atomic) mass Mr was introduced, which is defined as the ratio of the mass of a molecule (atom) of a given substance m0 to 1/12 of the mass of a carbon atom:
. (1)The number of molecules in the case of a one-component system containing molecules or atoms of the same type can be found by the formula:
where m is the mass of the system, expressed in kilograms.
From this formula it is clear that N has very large values, so it was necessary to introduce some relative parameter associated with the number of molecules N in the system, called the amount of substance.
IN International system units (SI), the mole is taken as such a parameter - the amount of a substance that contains the same number of molecules or atoms as there are atoms in 0.012 kg of carbon.
Thus, one mole of any substance contains the same number of molecules or atoms, which is called Avogadro’s number (constant) and is equal to:
.The amount of substance is defined as the number of moles equal to the ratio of the number of molecules N to Avogadro’s number:
. (2)The mass of one mole of a substance is called molar mass. It is equal to the product of the mass of one molecule of a substance m0 and Avogadro’s number:
and is measured in kilograms per mole: [M] = kg mol–1.
From (1), (3) and the definition of Avogadro’s number it follows that between the relative molecular weight substance Mr and its molar mass M there is a relationship:
M = Mr·10–3 kg·mol–1.
Considering equality
where m is the mass of the substance,
Using formulas (2) and (3), it is easy to obtain another expression for the amount of substance:
, (4)that is, the amount of a substance is equal to the ratio of the mass of this substance to its molar mass.
Thermal movement of molecules.
The disorder, chaotic movement of particles is the most important feature thermal movement. Experimental evidence of the continuous nature of the movement of molecules is diffusion and Brownian motion.
Diffusion is the phenomenon of spontaneous penetration of molecules of one substance into another.
As a result of the mutual diffusion of substances, a gradual equalization of their concentration occurs in all areas of the volume they occupy.
It has been established that the rate of the diffusion process depends on the type of diffusing substances and temperature. One of the most interesting phenomena that confirms the chaotic motion of molecules is Brownian motion. It represents the thermal movement of microscopic (but consisting of very large number molecules) particles of a substance suspended in a liquid or gas, first observed by R. Brown. The randomness of the movement of such particles is explained by the fact that the sum of the impulses they receive from molecules from different sides may become equal to zero as a consequence different numbers impacts from different sides of the particle, and as a result of the fact that the particle on one side could be hit by molecules with higher velocities than the molecules hitting it on the other side.
Brownian motion manifests itself more noticeably the more smaller particle and the viscosity of the medium, and the higher the temperature of the system. The dependence on temperature indicates that the speed of chaotic motion of molecules increases with increasing temperature, which is why it is called thermal motion.
Interaction of molecules.
Intermolecular forces are of an electromagnetic nature and come down to two types - attraction and repulsion. These forces are short-range and appear only at distances comparable to the size of molecules. The forces of attraction and repulsion quickly decrease with increasing distance between molecules, but the rate of their decrease is different. The repulsive force prevails at small distances and grows indefinitely as the distance between the centers of mass of the molecules r approaches a certain value d, which can be considered as the effective diameter of the molecules.
The attractive force decreases with increasing r much slower than the repulsive force, so there is a certain value of the intermolecular distance r = r0, at which the repulsive and attractive forces cancel each other out, so that the resulting intermolecular interaction force becomes zero.
Change state of aggregation substances
Aggregate states
Any substance can be in three states of aggregation: solid, liquid and gaseous.
In gases, the average kinetic energy of the thermal motion of molecules significantly exceeds the potential energy of their interaction. In this case, the interaction forces between molecules have very little effect on the nature of their relative motion, since the molecules are located at a fairly large distance from each other. As the temperature decreases or during compression, the interaction of molecules begins to play such a significant role that the gas eventually turns into a condensed state - a liquid.
In a liquid, the average interaction energy of molecules is approximately equal to the average energy of thermal motion. Thermal movement breaks the connection between molecules and leads to their movement relative to each other within the volume of liquid. In this regard, the liquid takes the shape of the vessel in which it is placed.
Solids usually mean crystals, characteristic feature which is the regular arrangement of atoms or ions in them. About the set of points at which they are located atomic nuclei, they talk about crystal lattice, and these points themselves are called lattice nodes.
The thermal motion of atoms or ions in a crystal is mainly vibrational in nature. However, since in a crystal the kinetic energy of the vibrational motion of atoms is significantly less than the absolute value potential energy their interaction, then thermal motion cannot destroy the bond between atoms. Therefore, a solid, unlike a liquid, retains its shape and has great mechanical strength.
Except crystalline bodies there are amorphous bodies. Although they are usually considered solids, they are supercooled liquids. If we consider a certain atom of an amorphous body as central, then the atoms closest to it will be located in a certain order, but as we move away from the “central” atom, this order is violated and the arrangement of atoms becomes random. Amorphous bodies include glass, plastics, etc.
The transition from one state of aggregation to another (at constant pressure) occurs at a strictly defined temperature and is always associated with the release or absorption of a certain amount of heat. The transition of a substance from one state to another does not occur instantly, but over a period of time, when two states of the substance exist simultaneously in thermal equilibrium.
Melting and crystallization
As the temperature increases, the energy of the vibrational motion of atoms in solid body increases and, finally, a moment comes when the bonds between atoms begin to break. In this case, the solid turns into a liquid state. This transition is called melting. At a fixed pressure, melting occurs at a strictly defined temperature.
The amount of heat required to convert a unit mass of a substance into a liquid at its melting point is called specific heat melting .
To melt a substance of mass m, it is necessary to expend an amount of heat equal to:
When a molten solid cools, reverse process called crystallization. Crystal formation also occurs at a constant temperature equal to the melting point. When a liquid crystallizes, the same amount of heat is released as is absorbed when a substance of the same mass melts.
Amorphous bodies, unlike crystals, do not have a specific melting point.
Evaporation and condensation.
Both in liquids and solids There is always a certain number of molecules whose energy is sufficient to overcome the attraction of other molecules and which are capable of breaking away from the surface of a liquid or solid and moving into the space surrounding them. This process for liquids is called evaporation (or vaporization), and for solids - sublimation (or sublimation).
The amount of heat Q that must be imparted to a liquid to evaporate a unit of its mass at a constant temperature is called the specific heat of vaporization r.
The amount of heat that must be expended to convert a liquid of mass m into vapor is
As a result of chaotic movement above the surface of the liquid, the vapor molecule, falling into the sphere of action of molecular forces, returns to the liquid again. This process is called condensation. Evaporation of a liquid occurs at any temperature and the faster the higher the temperature, the larger the free surface area of the evaporating liquid and the faster the vapors formed above the liquid are removed.
It should be noted that the process of vaporization is associated with an increase internal energy substances, and the process of condensation - with its decrease.
Saturated and unsaturated pairs. Humidity.
If during the same time the number of evaporating and condensing vapor molecules is the same, then the number of vapor molecules above the liquid will remain constant. This state is called dynamic equilibrium of vapor and liquid. Vapor that is in dynamic equilibrium with a liquid is called saturating (or saturated). At a constant temperature, the density of the saturating vapor above the liquid remains constant.
Steam whose density is less than the density of saturating steam at the same temperature is called non-saturated (or unsaturated).
Unsaturated steam obeys the laws of an ideal gas.
A special case of evaporation is boiling. This is a process of intense vaporization not only from the free surface, but also in the volume of the liquid. Bubbles filled with saturated steam form in the volume. They rise upward under the action of buoyant force and burst on the surface. The centers of their formation are tiny bubbles of foreign gases or particles of various impurities.
The process of turning a liquid into vapor requires energy to break the bonds between liquid molecules and to work against external pressure forces. Pressure saturated steam Pus inside a bubble located at the surface of the liquid is equal to the sum of the external pressure on the liquid Pvn and the pressure under the curved surface of the liquid.
Rnas= Rvn+ 2/r, (7)
where r is the radius of the bubble,
- surface tension coefficient.
If the bubble has dimensions of the order of several millimeters or more, then the second term can be neglected and, therefore, for large bubbles at constant external pressure, the liquid boils when the saturated vapor pressure in the bubbles becomes equal to the external pressure.
In order to judge whether there is a lot or little water vapor in the air, the concept of humidity is introduced. Absolute humidity is the amount of steam, expressed in kilograms, contained in 1 m3 at a given temperature, i.e. absolute humidity is equal to the vapor density of water. Relative humidity B is the ratio of absolute humidity to saturated vapor density at a given temperature.
100% . (8)The density of saturated water vapor at a given temperature is a tabular value. To determine relative humidity, you need to know absolute humidity, which can be determined by the dew point.
The dew point corresponds to the temperature at which the vapor in the air becomes saturated.
Molecular kinetic theory ideal gases
Thermal equilibrium.
Temperature. Celsius temperature scale.
Molecular physics and thermodynamics study the properties and behavior of macroscopic systems, i.e. systems consisting of a huge number of atoms and molecules. Typical systems that we encounter in Everyday life, contain about 1025 atoms.
When studying such systems, the most important are macroscopic quantities that are directly measured experimentally and characterize the properties of the entire set of molecules as a whole. Given the extraordinary complexity of macrosystems, one should begin studying with the simplest objects - systems whose state does not change over time. The state of a macroscopic system in which it can be indefinitely for a long time, is called equilibrium (it is also spoken of as a state of thermal equilibrium).
The equilibrium state of the system as a whole can be described using quantities called macroscopic parameters, which include pressure, volume, etc. Each parameter characterizes a certain property of the system. So volume V is a measure of the property of a system to occupy a particular region of space; pressure P is a measure of a system’s ability to resist external changes in its volume.
In a state of thermal equilibrium, macroscopic parameters do not change over time and remain constant.
One of the most important parameters characterizing the equilibrium properties of a macroscopic system is temperature. Let's introduce this parameter, for which we consider two bodies that can interact and exchange energy. This type of interaction, which is called thermal, leads to the fact that as a result of collisions of molecules in the area of contact of two bodies, energy is transferred from fast molecules to slow ones. This means that the energy of motion of atoms in one body decreases, in another it increases. The body that loses energy is called more heated, and the body to which the energy transfers is called less heated. This energy transfer continues until a state of thermal equilibrium is established. In a state of thermal equilibrium, the degrees of heating of bodies are the same. To characterize the degree of heating of a body, a parameter called temperature is introduced.
It is known from experience that when temperature changes, the dimensions of bodies, electrical resistance and other properties change. Thus, temperature can be determined by a change in any convenient measurement physical properties of this substance.
Most often, temperatures are measured using the property of a liquid to change volume when heated and cooled. The device with which temperature is measured is called a thermometer.
An ordinary liquid thermometer consists of a small glass reservoir to which is attached a glass tube with a narrow internal channel. The reservoir and part of the tube are filled with mercury or other liquid. The temperature of the medium in which the thermometer is immersed is determined by the position of the upper level of mercury in the tube. It was agreed to mark the divisions on the scale as follows. The number 0 is placed in the place of the scale where the level of the liquid column is established when the thermometer is lowered into melting snow, the number 100 is placed in the place where the level of the liquid column is established when the thermometer is immersed in water vapor boiling at normal pressure (105 Pa). The distance between these marks is divided by 100 equal parts, called degrees. This temperature scale was created by Celsius. Degrees on the Celsius scale are denoted °C.
In addition to macroscopic parameters, system parameters related to the individual characteristics of its constituent particles, called microscopic, are introduced. These include, first of all, the mass of particles, their speed, and kinetic energy.
Ideal gas. The basic molecular equation kinetic theory ideal gas.
The theory was created by the German physicist R. Clausis in 1957 for a model of a real gas called an ideal gas. Main features of the model:
the distances between molecules are large compared to their sizes;
there is no interaction between molecules at a distance;
When molecules collide, large repulsive forces act;
The collision time is much less than the time of free movement between collisions.
Molecular kinetic theory (MKT) establishes connections between the macro- and microparameters of an ideal gas. The basic MKT equation expresses the relationship between gas pressure and the average kinetic energy of the translational motion of molecules. The pressure of the gas on the walls of the container is the result of numerous impacts of molecules. With each impact, the wall receives a force impulse, the magnitude of which depends on the speed of the molecules and, therefore, on the energy of their movement. With a huge number of impacts, a constant gas pressure is created on the wall. The number of impacts depends on the concentration of molecules n. Thus, we can expect that gas pressure is related to the concentration of molecules and the energy of their movement. We obtain the basic MKT equation.
Consider a spherical volume of radius R containing N molecules of an ideal gas. Let's consider the movement of one of them. Let us assume that the molecule moved rectilinearly with momentum
hit the wall at an angle ψ to the normal and bounced off it at the same angle, having momentum . Let's find the momentum transferred by the molecule to the wall upon impact. From the law of conservation of momentum:Because the impact is elastic,
and = 0, therefore directed normal to the wall and modulo equal to: .The path that a molecule travels from one impact on the wall to another is equal to the chord AB, i.e., the value 2Rcosψ.
Let's find the number of times a molecule hits a wall in one second. It is equal to the ratio of the speed of the molecule
to the path traversed by a molecule from one collision with a wall to another: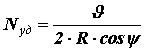 .
.
Colliding with the walls of a vessel, one molecule imparts momentum to it in one second
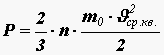
The total impulse imparted by all N molecules to the wall of the vessel in one second will be equal to
.From Newton’s II law it follows that the impulse imparted per unit time to the wall is numerically equal to the force, therefore the pressure force acting on the surface of the vessel is equal to
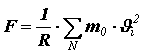 .
.
We find the pressure by dividing the force by the surface area of the spherical vessel:
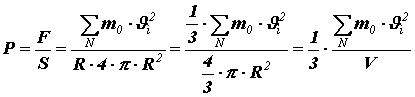 , – volume of the vessel with gas.
, – volume of the vessel with gas. Let us rewrite the resulting equality as:
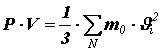
Multiplying and dividing the right side by the number of molecules N in volume V, we get:
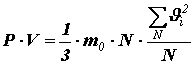 or
or The value entered here
– root mean square speed, equal to the square root of the sum of the squares of all speeds divided by the number of molecules: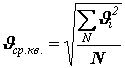 . (10)
. (10)
This equation is called the basic equation of the molecular kinetic theory of an ideal gas.
Let us obtain a relationship between pressure and the average kinetic energy of the translational motion of a molecule
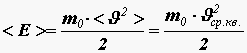 . (12)
. (12)
From formula (11)
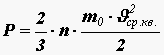 , hence:
, hence: Thus, the pressure of an ideal gas is proportional to the product of the concentration of molecules and the average kinetic energy of the translational motion of the molecule. This statement can be considered another formulation of the basic equation of the molecular kinetic theory of an ideal gas.
Dalton's law.
Consider a gas consisting of molecules various substances, which is located in volume V. Due to the chaotic thermal motion, the molecules of each component of the mixture will be distributed evenly throughout the volume, i.e. as if the remaining components of the gas were absent. Due to the constant collisions of molecules with each other, accompanied by a partial exchange of impulses and energies between them, thermal equilibrium is established in the mixture. All this leads to the fact that the pressure of each component of the mixture will not depend on the presence of the others.
Then the resulting pressure is determined by the total pressure of all components, i.e. Dalton's law is valid for a mixture of gases: the pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the gases entering it.
, (14)where k is the number of the gas component in the mixture, Pk is its partial pressure, i.e. the pressure that the kth gas would have if it alone occupied the entire volume occupied by the mixture.
Mean square speed of molecules.
From the basic equation of molecular kinetic theory, one can obtain a formula for calculating the root mean square speed of molecules
The product of the mass of one molecule m0 by the number of molecules per unit volume n is equal to the density of the substance r:=r m0×n.
Thus,
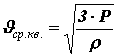 . (15)
. (15)
experimental gas laws
Isoprocesses. Laws of Boyle-Mariotte, Gay-Lussac, Charles.
Any change in the state of a gas is called a thermodynamic process.
The simplest processes in an ideal gas are isoprocesses. These are processes in which the mass of a gas and one of its state parameters (temperature, pressure or volume) remain constant.
An isoprocess that occurs at a constant temperature is called isothermal.
Experimentally, R. Boyle and E. Mariotte found that at a constant temperature, the product of gas pressure and volume for a given mass of gas is a constant value (Boyle–Mariotte law):
Graphically, this law in PV coordinates is depicted by a line called an isotherm (Fig. 1).
An isoprocess occurring in an ideal gas, during which the pressure remains constant, is called isobaric.
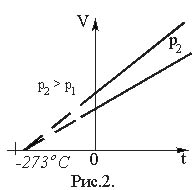
The dependence of the volume of a gas on its temperature at constant pressure was established by L. Gay-Lussac, who showed that the volume of a gas of a given mass at constant pressure increases linearly with increasing temperature (Gay-Lussac’s law):
V = V0·(1 + ·t), (17)
where V is the volume of gas at temperature t, °C; V0 is its volume at 0°C.
Magnitude
called the temperature coefficient of volumetric expansion. For all gases = (1/273°С–1). Therefore ·t). (18)Graphically, the dependence of volume on temperature is depicted by a straight line – an isobar (Fig. 2). At very low temperatures (close to – 273°C), the Gay-Lussac law is not satisfied, so the solid line on the graph is replaced by a dotted line.
An isoprocess occurring in a gas in which the volume remains constant is called isochoric.
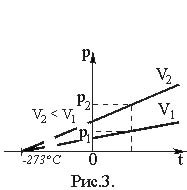
Studies of the dependence of the pressure of a given mass of gas on temperature at a constant volume were first carried out by the French physicist Charles. He found that the pressure of a gas of a given mass at a constant volume increases linearly with increasing temperature (Charles’ law):
t). (19)Here P is the gas pressure at temperature t, °C; P0 is its pressure at 0 °C.
Magnitude
called the temperature coefficient of pressure. Its value does not depend on the nature of the gas; for all gases = 1/273 °C–1. Thus ·t). (20)The graphical dependence of pressure on temperature is depicted by a straight line - an isochore (Fig. 3).
Absolute temperature scale.
Fundamentals of molecular kinetic theory
The main position of the molecular kinetic theory is the statement that all bodies consist of tiny particles (molecules, atoms, etc.) that move and interact with each other. Evidence molecular structure substances are fragmentation of bodies, melting, evaporation, diffusion, Brownian motion, etc.
The molar mass M of a substance is the mass of the number of molecules of a given substance contained in carbon 12 C weighing 12 g. The molar mass of a substance can be found from the periodic table by adding atomic masses all the atoms that make up the molecule of this substance. Wherein molar mass will be measured in g/mol. To convert to the SI system, this value should be multiplied by 10 -3. In this case, the molar mass is measured in kg/mol. For example, the molar mass of hydrogen H 2 is 2 g/mol = 2⋅10 3 kg/mol.
One mole of any substance contains N A = 6.022⋅10 23 mol -1 molecules. The number N A is called Avogadro's constant. The mass of one molecule m0 is expressed by the formula
The amount of substance v is the ratio of the number of molecules N to Avogadro’s number N A:
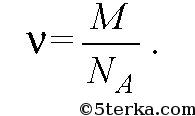
If m is the mass of the substance, then
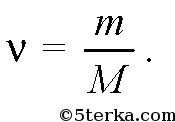
An ideal gas is a gas in which molecules move freely and interact with each other and with the walls of the container only through collisions. The ideal gas model satisfactorily describes fairly rarefied gases.
RMS speed of molecules
the following physical quantity is called
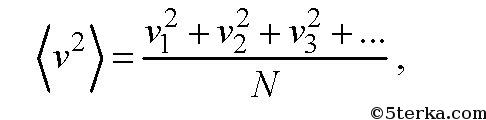
where v 1, v 2, v 3,... are the speeds of molecules: first, second, third, and so on up to N. Note that the average speed of molecules is zero and not equal
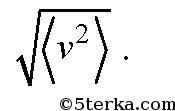
The concentration of molecules n is the ratio of the number of molecules N in volume V to this volume V:
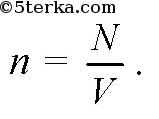
The pressure p can be expressed by the following formula
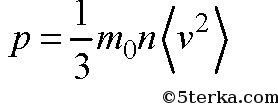
This equation is called the basic equation of the molecular kinetic theory (MKT) of gases. This equation can be rewritten as
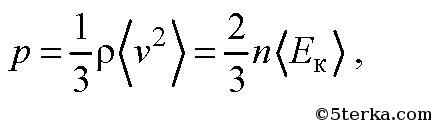
where ρ is the gas density,
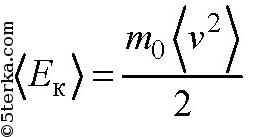
Average kinetic energy of a gas molecule. Average kinetic energy
is related to the gas temperature T by the formula
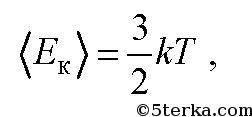
Where k-Boltzmann constant. It is numerically equal
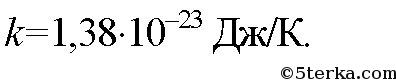
The following formula can be proven:
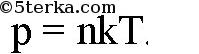
It follows from the Mendeleev-Clapeyron equation
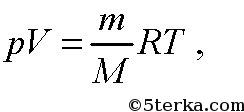
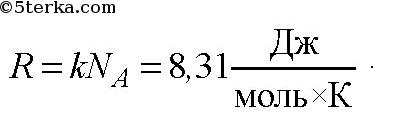
Universal gas constant.
With constant gas mass and composition
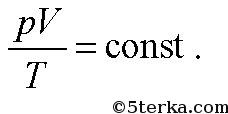
If the temperature is also constant, then
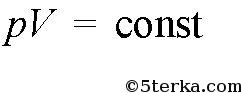
(isothermal process), if the pressure is constant, then

(isobaric process), if the volume is constant, then
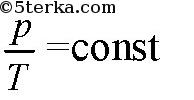
(isochoric process).
Water vapor is always present in the Earth's atmosphere as a small impurity, but it largely determines the weather. Air humidity can be characterized by partial vapor pressure p or vapor density ρ (absolute humidity). Saturated steam is steam that is in dynamic equilibrium with its liquid. At a certain temperature, there is a pressure at which water vapor becomes saturated. This pressure p us is called saturated vapor pressure. This pressure can be found in the table in the problem book. Relative humidity φ is the ratio of the partial vapor pressure p to the saturated vapor pressure
In this lesson we will derive the basic equation of molecular kinetic theory (MKT), which connects the macroparameters of a gas with the microparameters of individual molecules.
Let us recall the basic information about the ideal gas model:
Molecules move chaotically;
The mechanism of ideal gas pressure is the collision of individual molecules with the walls of the vessel.
Let an ideal gas be in a cylindrical vessel (see Fig. 1). Let's determine the pressure p this gas to the piston.
Rice. 1. Ideal gas (molecules) in a cylindrical vessel
By definition, pressure is a quantity equal to the ratio of force ( F), acting perpendicular to the surface, to the area of this surface ( S).
Let's calculate the force ( F), with which the molecules act on the piston:
1. Determine the force of impact of one molecule on the wall of the vessel.
Let an ideal gas molecule of mass move in the plane XOY with speed and, having hit the piston, rebounds from it with speed (see Fig. 2). According to Newton's second law, the force exerted on a molecule by the piston during impact is equal to:
![]() ,
,
Where a- acceleration of a molecule upon impact; - change in the speed of movement of the molecule upon impact; - duration of impact.
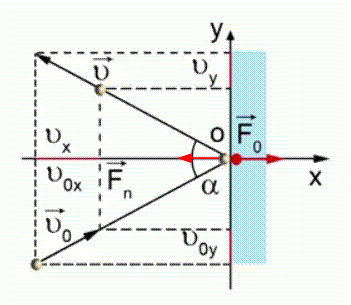
Rice. 2. Collision of a molecule with a piston
Projection of speed onto the axis OY does not change, so the entire change in speed is equal to the change in speed along the axis X:
According to Newton's third law, the force with which the molecule acts on the piston is equal in magnitude to the force with which the piston acts on the molecule. Hence:
2. Calculate the number of molecules N, hitting the piston during the interval.
During the time interval, only those molecules that move in the direction of the piston and are distant from it will have time to reach the piston (see Fig. 3). That is, in fact, half the number of molecules enclosed in a cylinder with a volume ![]() . Therefore, the number of molecules hitting the piston during the interval is equal to:
. Therefore, the number of molecules hitting the piston during the interval is equal to:
The total number of molecules, which is equal to the product of concentration and volume:
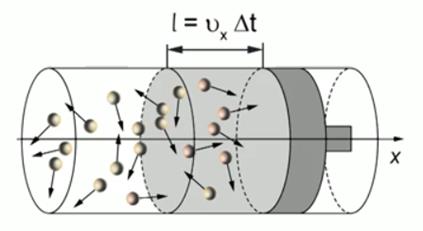
Rice. 3. Molecules hitting the piston over time
3. Let us determine the total force of impacts of molecules on the piston.
This force will be equal to the product of the impact force of one molecule times total number beats:
We live in a three-dimensional world, that is, any molecule has a velocity projection. Since all molecules move chaotically, the directions of their motion are equal, so we can write that on average, for the mean square speed, they are the same (). Therefore, we replace the square of the velocity projection with the mean square of the velocity projection:
![]()
We substitute this value into the formula for the force of impacts of molecules on the piston:
Let us substitute the value of this force into the pressure formula:
![]()
- basic equation of MKT ideal gas,
- the pressure of an ideal gas is equal to two-thirds of the average kinetic energy of the translational motion of the molecules contained in a unit volume
In this lesson we derived the basic MKT equation. We will refer to this equation infrequently, since it is more convenient to work with individual macroparameters (it is easier to separately measure pressure, volume, temperature than to measure the speed and mass of a specific molecule). Nevertheless, it was this equation that was called the main one, because it provides a connection between the macrocosm and the microcosm.
Bibliography
G.Ya. Myakishev, B.B. Bukhovtsev, N.N. Sotsky. Physics 10. - M.: Education, 2008.
Physics. Tests. Grades 10-11: educational and methodological manual / N.K. Gladysheva, I.I. Nurminsky, A.I. Nurminsky and others - M.: Bustard, 2005
Gendenshtein L.E., Dick Yu.I. Physics 10th grade. - M.: Ilexa, 2005.
Kasyanov V.A. Physics 10th grade. - M.: Bustard, 2010.
- Easy-physics.ru ().
- Clck.ru ().
- Clck.ru ().
Homework
Its molecules.
The derivation of the basic equation of the molecular kinetic theory is based on the assumptions of the ideal gas model and the statement: gas pressure is the result of impacts of molecules on the wall of the vessel.
Let us determine the gas pressure on the wall with area S vessel ABCD.
Each molecule weighs m 0
, bouncing off the wall after an elastic collision with the wall, transfers momentum to it 2m 0 v x, Where v x— projection of the speed of the molecule onto the axis Oh, perpendicular to the wall. In just one second, the total momentum received by the wall from all molecules is equal to 2m 0 v x Z, Where Z— the number of such collisions (in 1 s) of all molecules. It's obvious that 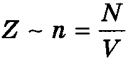 , Where n— concentration of molecules per unit volume; N- the number of all molecules. Number Z also proportional to the speed of molecules v x and wall area S: Z~ nv x S. Since all directions during the chaotic movement of gas molecules are equally probable, then of all the molecules that have a velocity component v x, only half moves towards the wall CD, second half - to the side AB(i.e. in reverse). That's why
, Where n— concentration of molecules per unit volume; N- the number of all molecules. Number Z also proportional to the speed of molecules v x and wall area S: Z~ nv x S. Since all directions during the chaotic movement of gas molecules are equally probable, then of all the molecules that have a velocity component v x, only half moves towards the wall CD, second half - to the side AB(i.e. in reverse). That's why 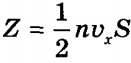 , and the total impulse transferred to the wall in 1 s is equal to 2m 0 v x Z = m 0 nv x 2 S. Since the change in the momentum of a point (body) per unit time is equal to the force acting on it
, and the total impulse transferred to the wall in 1 s is equal to 2m 0 v x Z = m 0 nv x 2 S. Since the change in the momentum of a point (body) per unit time is equal to the force acting on it 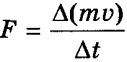 . In reality, since we are talking about a large number of molecules moving at different speeds, the force should be averaged:
. In reality, since we are talking about a large number of molecules moving at different speeds, the force should be averaged: ![]() .
.
This force thus depends on the mean square of the velocity. Since, due to the randomness of movement, all directions are equal, then:
![]()
On the other hand, it is known that the squared modulus of any vector is equal to the sum of the squares of its projections on the coordinate axes, therefore:
![]()
Averaging this expression over all molecules and taking into account , we obtain:
From here:
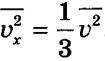
Taking into account the last formula 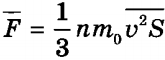 .
.
Therefore, the pressure on the wall of the vessel is equal to:
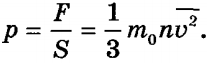
This is the basic equation of molecular kinetic theory. This equation is the first quantitative relationship obtained in molecular kinetic theory. The equation allows us to obtain a relationship between pressure and the average kinetic energy of molecules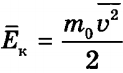 .
.
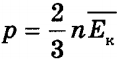 .
.
The pressure of an ideal gas is equal to two-thirds of the average kinetic energy of the translational motion of the molecules contained in a unit volume.
It is important to emphasize that here we are talking about the average kinetic energy of gas molecules. This means that gas pressure is a value organically related to the fact that the gas consists of a large number of molecules. There is no point in talking about pressure created by several molecules. Gas pressure is a concept of a statistical nature (this is the name given to concepts that make sense only for systems with a very large number of particles).
According to the ideal gas model, molecules are constantly in random motion, colliding with each other and with the walls of the vessel in which they reside. At the moment a molecule collides with a wall, it acts on it with a force, the momentum of which, according to Newton’s second law, is equal to the change in its momentum (amount of motion): F̅t =Δ(m͞ v). This means that the change in the modulus of the momentum of all molecules Σ Δ(mv i) upon impact is equivalent to the action of the average value of force F̅ for a time t. This combined action of the molecules causes gas pressure, which by definition is equal to p = F/S.
Gas pressure cause impacts on the wall of the vessel of a large number of molecules transmitting an impulse to it.
Let's consider an ideal gas located in a vessel with a volume V. For simplicity of calculations, we will choose it in the form of a parallel-piped (Fig. 1.15). Let it be in it N molecules weighing m 0 each; their concentration is equal to n= N/V. Since the molecules move randomly (the condition of dynamic chaos for an ideal gas), it is enough to determine the pressure on one of the walls, since the molecules will create the same pressure on the other walls. For simplicity of presentation, we will first assume that they all have the same speeds V.
Let us imagine a separate layer of gas perpendicular to the coordinate planeZY.Due to the randomness of the movement, the number of molecules flying into this layer from the right is equal to the number of molecules flying out of it from the left. It is obvious that both of them transmit impulse to this layer: those flying in from the left (Fig. 1.16) carry impulsem 0vx, Where v x —projection of speed onto the axisX; those flying out of it carry an impulse -m 0vx,overall giving him the impetus2 m 0vx. So, from the side of all moleculesZflying into a separate layer, the wall receives an impulse2 Zm 0vx.
Number of molecules Z, which collide with a wall of area S during t, can be determined by their concentration in the volume of a separate layer: Z=nv x tS, Where n — concentration of molecules. Since only those molecules reach the wall that have a positive velocity projection onto the axis X(v x> 0), then their number will be half the number of molecules crossing the plane of a separate gas layer:
Z=nv x tS / 2.
So, from all the molecules located in a separate layer, the wall receives a common impulse:
F x t = 2 . nv x tSm 0 v x / 2.
Dividing the left and right sides of the equality into St, we get:
F x /S=p =nm 0v 2x.
The assumption that the speeds of all molecules are the same was made in order to simplify the derivation of the equations. In fact, the range of their values is quite wide - from 0 to a certain maximum value vmax. Therefore, in the previous equation, to determine gas pressure, it would be more correct to take the average square of the velocity projection vx. Then it will look like:
p =nm 0v̅ 2 x.
It is clear that similar considerations will be valid for walls lying in other coordinate planes:
p = nm 0 v̅ 2 y ,
p =nm 0v̅ 2z.
The mean square of speed has the meaning of the statistical average value of speed.Material from the site
It is obvious that due to the chaotic movement of molecules v̅ 2x =v̅ 2y =v̅ 2z. By mathematical definition, the average square of the speed is equal to v̅ 2 = (v̅ 2x + v̅ 2y +v̅ 2z). From here v̅ 2x = (1 / 3) . v̅ 2. Substituting this expression into the equation p =nm 0v̅ 2 x, we get the final equation for determining ideal gas pressure:
p = (1 / 3). nm 0 v̅ 2 .
This formula is the basic equation of molecular kinetic theory (MKT) ideal gas, which determines the relationship between the macroparameter of the thermodynamic system - the pressure of the ideal gas and the characteristics of its microscopic state. Thus, it determines gas pressure as a statistical quantity through the microparameters of the system - concentration, mass and speed of the molecule.
Because the nm 0 = ρ , Where ρ — gas density, basic equation of molecular kinetic theory an ideal gas will also have the following form:
p = (1 / 3) . ρv̅ 2 .
Basic MKT equation is a bridge between two approaches to the interpretation of thermal phenomena and processes - thermodynamic and molecular-kinetic.
On this page there is material on the following topics:
Ideal gas lecture basic equation mkt
How does the momentum of a gas layer change due to molecules flying into it?
