Let us consider the dependence of some properties of atoms on the structure of their electronic shells. Let us dwell, first of all, on the patterns of changes in atomic and ionic radii.
Electron clouds do not have sharply defined boundaries. Therefore, the concept of the size of an atom is not strict. But if you imagine atoms in crystals simple substance in the form of balls touching each other, then the distance between the centers of neighboring balls (i.e., between the nuclei of neighboring atoms) can be taken equal to twice the radius of the atom. Thus, the smallest internuclear distance in copper crystals is equal to; this allows us to assume that the radius of the copper atom is equal to half of this value, i.e.
The dependence of atomic radii on the charge of the nucleus of an atom Z is periodic. Within one period, as Z increases, a tendency toward a decrease in atomic size appears, which is especially clearly observed in short periods (atomic radii are given in nm):
This is explained by the increasing attraction of electrons from the outer layer to the core as its charge increases.
With the beginning of the construction of a new electronic layer, more distant from the nucleus, i.e., during the transition to the next period, atomic radii increase (compare, for example, the radii of fluorine and sodium atoms). As a result, within a subgroup, with increasing nuclear charge, the sizes of atoms increase. Let us give as an example the values of the atomic radii (in nm) of elements of some main subgroups:

The electrons of the outer layer, which are least tightly bound to the nucleus, can be detached from the atom and attached to other atoms, becoming part of the outer layer of the latter.
Atoms that have lost one or more electrons become positively charged because the charge on the atomic nucleus exceeds the sum of the charges on the remaining electrons. On the contrary, atoms that have added extra electrons become negatively charged. The charged particles produced are called ions.
Ions are designated by the same symbols as atoms, indicating their charge at the top right: for example, a positive triply charged aluminum ion is denoted by , a negative singly charged chlorine ion is denoted by .
The loss of electron atoms leads to a decrease in its effective size, and the addition of excess electrons leads to an increase. Therefore, the radius of a positively charged ion (cation) is always smaller, and the radius of a negatively charged non (anion) is always greater than the radius of the corresponding electrically neutral atom. Thus, the radius of the potassium atom is , and the radius of the ion, the radii of the chlorine atom and ion, respectively, are 0.099 and . In this case, the radius of the ion differs more strongly from the radius of the atom, the greater the charge of the ion. For example, the radii of the chromium atom and ions are 0.127, 0.083 and , respectively.
Within one subgroup, the radii of ions of the same charge increase with increasing nuclear charge. This is illustrated by the following examples (ion radii are given in nm):

This pattern is explained by an increase in the number of electronic layers and the growing distance of outer electrons from the nucleus.
An atom is an electrically neutral system consisting of a positively charged nucleus and negatively charged electrons.
The nuclei of atoms consist of two types of particles (nucleons) - protons (p) and neutrons (n). The charge of a proton is equal in magnitude and opposite in sign to the charge of an electron; its mass is approximately one amu. A neutron is an uncharged particle with a mass of approximately equal mass proton.
The linear dimensions of an atom are ~10 -8 cm, those of a nucleus are ~10-12 -10 -13 cm.
The bulk of the atom is concentrated in the nucleus and is characterized by a mass number A equal to the sum of the numbers of protons (nuclear charge) Z and neutrons N: A=Z+N.
The main characteristic of an atom is the charge of the nucleus (Z). It determines the number of electrons around the nucleus, i.e. belonging of an atom to a given type of chemical element, and corresponds to the atomic number (in periodic table elements - serial number) of the element.
The designation of an element's atom reflects the mass number and number of protons - for example.
Relative atomic mass of an element is the average value of the mass numbers of its natural isotopes, taking into account the degree of their distribution. For example, chlorine in nature is found mainly in the form of two isotopes - (75.43%) and (24.57%). The relative atomic mass of chlorine is  .
.
basis modern theory The structure of the atom is the laws and provisions of quantum (wave) mechanics - a branch of physics that studies the movement of micro-objects.
Microobjects have both corpuscular and wave properties. To describe the movement of microparticles, a probabilistic approach is used, that is, not their exact position is determined, but the probability of being in a particular region of the perinuclear space.
The state of an electron in an atom is described using a quantum mechanical model - an electron cloud, the density of the corresponding sections of which is proportional to the probability of finding an electron. Usually, the electron cloud is understood as the region of the perinuclear space, which covers approximately 90% of the electron cloud. This region of space is also called an orbital.
There is a system of quantum numbers that determines the state of an electron in an atom.
Principal quantum number n determines the energy of the electron and the size of the electron cloud. It can take integer values from 1 to .
A set of electronic states that have the same principal quantum number n, is called the electron layer or energy level.
Lowest value energy E corresponds n=1. The remaining quantum states correspond to higher energy values. Electrons found at these energy levels are less tightly bound to the nucleus.
For the hydrogen atom, the quantum state with n=1 corresponds to its lowest energy and is called fundamental. States n= 2, 3, 4... are called excited.
Orbital The (side) quantum number determines the orbital angular momentum of the electron and characterizes the shape of the electron cloud. It accepts all integer values from 0 to ( n-1). To each n corresponds to a certain number of values, that is, the energy level is a set of energy sublevels, slightly different in energy. The number of sublevels into which the energy level is split is equal to the level number(that is, the numerical value n). These sublevels have the following letter designations:
Orbital quantum number: 0 1 2 3 4
Sublevel: s p d f g
The shapes of the orbitals corresponding to different values of are shown in the following figure.
Plasma).
Properties of the atom, including the most important ability of the atom to form chemical compounds, are determined by the features of its structure.
General characteristics of the structure of the atom. An atom consists of a positively charged nucleus surrounded by a cloud of negatively charged electrons. The dimensions of an atom as a whole are determined by the dimensions of its electron cloud and are large compared to the dimensions of the nucleus (the linear dimensions of an atom are ~ 10~8 cm, its nucleus ~ 10" -10" 13 cm). The electron cloud of an atom does not have strictly defined boundaries, therefore the dimensions of an atom are largely arbitrary and depend on the methods for determining them. The nucleus of an atom consists of Z protons and N neutrons held by nuclear forces. Positive proton charge and negative. the electron charge is identical in absolute value and equal to e = 1.60*10 -19 C; A neutron has no electrical charge. Nuclear charge +Ze is the main characteristic of an atom, which determines its belonging to a certain chemical element. Ordinal number of the element in the periodic table of Mendeleev (atomic number) equal to the number in the core.
In an electrically neutral atom, the number in the cloud is equal to the number of protons in the nucleus. However, under certain conditions it can lose or gain electrons, becoming positive or negative, respectively. for example Li +, Li 2+ or O -, O 2-. When talking about atoms of a certain element, we mean both neutral atoms and that element.
The mass of an atom is determined by the mass of its nucleus; The mass of an electron (9.109 * 10 -28 g) is approximately 1840 times less than the mass of a proton or neutron (1.67 * 10 -24 g), so the contribution to the mass of the atom is insignificant. Total number And A = Z + N called . The mass number and nuclear charge are indicated respectively by superscripts and subscripts to the left of the element symbol, for example 23 11 Na. View of atoms of one element with a specific value N called . Atoms of the same element with the same Z and different N called of this element The difference in the masses of isotopes has little effect on their chemical and physical properties Oh. The most significant differences are observed in hydrogen isotopes due to the large relative difference in the masses of ordinary (protium), D and T. The exact values of atomic masses are determined by mass spectrometry methods.
Atom. Due to its small size and large mass, the nucleus of an atom can be approximately considered pointlike and at rest in the center of mass of the atom, and the atom can be considered as a system of electrons moving around a stationary center - the nucleus. The total energy of such a system E equal to the sum of kinetic energies T everyone and potential energy U, which consists of the energy of attraction by the nucleus and the energy of mutual repulsion of electrons from each other. The atom obeys the laws of quantum mechanics; its main characteristic as a quantum system is the total energy E - can take only one of the values of a discrete series E 1 ...; intermittent An atom cannot have energy values. Each of the "allowed" values E corresponds to one or more stationary (with energy that does not change over time) states of the atom Energy E can only change abruptly - through a quantum transition of an atom from one stationary state to another. Quantum mechanics methods can be used to accurately calculate E for one-electron atoms - hydrogen and hydrogen-like: E= -hcRZ 2 /n 2, Where h- Planck's constant With- speed of light, integer P= 1, 2, 3, ... defines discrete energy values and called. principal quantum number; Rydberg constant ( hcR = 13.6 eV). When using SI, the formula for expressing discrete energy levels of one-electron atoms is written as:

Where t e - electron mass, is an electrical constant. Possible “allowed” energy values in an atom are depicted in the form of a diagram of energy levels - horizontal straight lines, the distances between which correspond to the differences between these energy values (Figure 1). the lowest level of E 1, corresponding to the minimum possible energy, is called ground, all others are called excited. The states (ground and excited) to which the indicated energy levels correspond are called similarly. With growth P the levels come closer and when the electron energy approaches the value corresponding to a free (resting) electron removed from the atom. Quantum state of an atom with energy E fully described wave function, where r is the radius vector of the electron of the nucleus The product is equal to the probability of finding an electron in the volume dV, that is, the probability density. The wave function is determined by the Schrödinger equation = , where the R operator total energy(Hamiltonian).
Along with energy, the motion of an electron around the nucleus (orbital motion) is characterized by orbital angular momentum (orbital mechanical momentum) M 1 ; the square of its magnitude can take values determined by the orbital quantum number l = 0, 1, 2, ...; ![]() , Where . For a given and the quantum number l can take values from 0 to (and - 1). The projection of the orbital momentum onto a certain z axis also takes on a discrete series of values M lz = , where m l is a magnetic quantum number having discrete values from - l to +l(-l,... - 1, 0, 1, ... + l), total 2l+ 1 values. The z axis for an atom in the absence of external forces is chosen arbitrarily, and in a magnetic field it coincides with the direction of the field strength vector. The electron also has its own angular momentum -spin and the associated spin magnetic moment. Squared spin mechanical moment M S 2 = S(S+ + 1) is determined by the spin quantum number S= 1/2, and the projection of this moment onto the z axis M sz = =-quantum number m s, taking half-integer values m s = 1 / 2 And m s= - 1 / 2 .
, Where . For a given and the quantum number l can take values from 0 to (and - 1). The projection of the orbital momentum onto a certain z axis also takes on a discrete series of values M lz = , where m l is a magnetic quantum number having discrete values from - l to +l(-l,... - 1, 0, 1, ... + l), total 2l+ 1 values. The z axis for an atom in the absence of external forces is chosen arbitrarily, and in a magnetic field it coincides with the direction of the field strength vector. The electron also has its own angular momentum -spin and the associated spin magnetic moment. Squared spin mechanical moment M S 2 = S(S+ + 1) is determined by the spin quantum number S= 1/2, and the projection of this moment onto the z axis M sz = =-quantum number m s, taking half-integer values m s = 1 / 2 And m s= - 1 / 2 .

Rice. 1. Diagram of hydrogen energy levels (horizontal lines) and optical transitions (vertical lines). Below is part of the atomic spectrum of hydrogen emission - two series of spectral lines; The dotted line shows the correspondence of lines and transitions of the electron
The stationary state of a one-electron atom is uniquely characterized by four quantum numbers: n, l, m l and m s. The energy of a hydrogen atom depends only on n, and level with a given n corresponds to a number of states differing in the values of l, m l, m s. States with given n and l is usually denoted as 1s, 2s, 2p, 3s etc., where the numbers indicate the values of k, and the letters s, p, d, f and further in the Latin alphabet correspond to the values l = 0, 1, 2, 3, ... Number of different states with given n and l is equal to 2(2l+ 1) the number of combinations of values m l and m s . Total number of divers. states with a given n equals 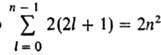 , i.e. levels with values P= 1, 2, 3, ... corresponds to 2, 8, 18, ..., 2n 2 decomp. quantum states. A level to which only one quantum state (one wave function) corresponds is called non-degenerate. If a level corresponds to two or more quantum states, it is called degenerate. In the hydrogen atom, the energy levels are degenerate in the values of l and m l; degeneracy in m s occurs only approximately, if we do not take into account the interaction of the spin magnetic moment electron with magnetic field, caused by the orbital motion of the electron in the electric field of the nucleus. This is a relativistic effect, small in comparison with the Coulomb interaction, but it is fundamentally significant, since it leads to additional splitting of energy levels, which manifests itself in atomic spectra in the form of the so-called fine structure.
, i.e. levels with values P= 1, 2, 3, ... corresponds to 2, 8, 18, ..., 2n 2 decomp. quantum states. A level to which only one quantum state (one wave function) corresponds is called non-degenerate. If a level corresponds to two or more quantum states, it is called degenerate. In the hydrogen atom, the energy levels are degenerate in the values of l and m l; degeneracy in m s occurs only approximately, if we do not take into account the interaction of the spin magnetic moment electron with magnetic field, caused by the orbital motion of the electron in the electric field of the nucleus. This is a relativistic effect, small in comparison with the Coulomb interaction, but it is fundamentally significant, since it leads to additional splitting of energy levels, which manifests itself in atomic spectra in the form of the so-called fine structure.
For given n, l and m l, the square of the modulus of the wave function determines the average distribution of electron density for the electron cloud in the atom. Different quantum states of the hydrogen atom differ significantly from each other in the distribution of electron density (Fig. 2). Thus, at l = 0 (s-state), the electron density is nonzero at the center of the atom and does not depend on the direction (i.e., it is spherically symmetric); for other states it is equal to zero at the center of the atom and depends on the direction.
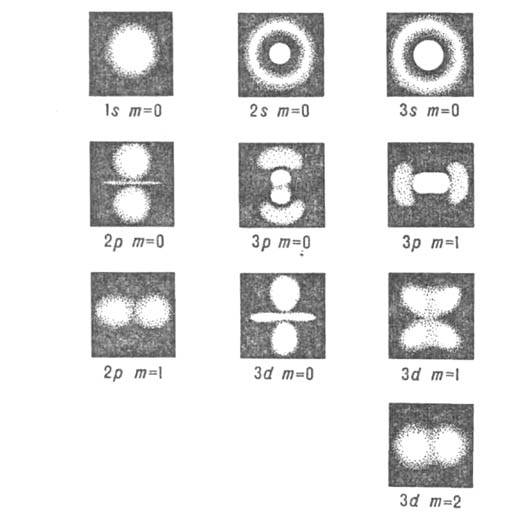
Rice. 2. Shape of electron clouds for different states of hydrogen
In multielectron atoms, due to the mutual electrostatic repulsion of electrons, the strength of their bond with the nucleus significantly decreases. For example, the energy of electron removal from . He + is equal to 54.4 eV, in a neutral He atom it is much less - 24.6 eV. For heavier atoms, the bond between the outer electrons and the nucleus is even weaker. An important role in multielectron atoms is played by specific exchange interaction, associated with the indistinguishability of electrons, and the fact that electrons obey the Pauli principle, according to which each quantum state characterized by four quantum numbers cannot contain more than one electron. For a many-electron atom, it makes sense to talk only about the quantum states of the entire atom as a whole. However, approximately, in the so-called one-electron approximation, it is possible to consider individual quantum states and characterize each one-electron state (a certain orbital, described by the corresponding function) by a set of four quantum numbers n, l, m l and m s. Collection 2(2l+ 1) in state with data n and l forms an electronic shell (called sdftve. also a sublevel, subshell); if all these states are occupied by electrons, the shell is called filled (closed). Totality 2n 2 states with the same n, but different l forms an electronic layer (also called a level, shell). For n= 1, 2, 3, 4, ... layers are indicated by symbols TO, L, M, N,... The number of electrons in shells and layers when completely filled are given in the table:
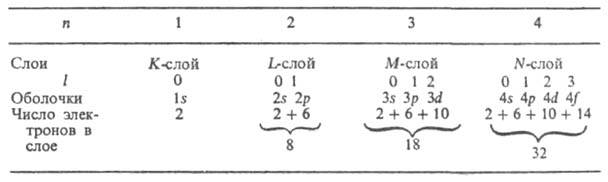
The bond strength of an electron in an atom, that is, the energy that must be imparted to an electron in order to remove it from the atom, decreases with increasing n, and for a given n - s increasing l. The order in which electrons fill shells and layers in a complex atom determines its electronic configuration, that is, the distribution of electrons among shells in the ground (unexcited) state of this atom and its . With this filling, electrons with increasing values of u and / are sequentially associated. For example, for the nitrogen atom (Z = 7) and its N + , N 2+ , N 3+ , N 4+ , N 5+ and N 6+ electronic configurations are respectively: Is 2 2s 2 2p 3 ; Is 2 2s 2 2p 2 ; Is 2 2s 2 2p; Is 2 2s 2 ; Is 2 2s; Is 2; Is (the number of electrons in each shell is indicated by the index at the top right). Neutral atoms of elements with the same number of electrons have the same electronic configurations as that of nitrogen: C, B, Be, Li, He, H (Z = 6, 5, 4, 3, 2, 1). Starting from n = 4, the order of filling the shells changes: electrons with a larger n, but with smaller l they turn out to be bound more tightly than electrons with smaller n and large l (Klechkovsky’s rule), for example, 4s electrons are bound more tightly than 3d electrons, and the 4s shell is filled first, and then 3d. When filling the shells 3d, 4d, 5d groups of corresponding transition elements are obtained; when filling 4f- and 5 f-shells - resp. lanthanides and actinides. The filling order usually corresponds to an increasing sum of quantum numbers ( n+l ); if these sums are equal for two or more shells, the shells with the smaller and are filled first. The following takes place. sequence of filling electron shells:

For each period are indicated electronic configuration noble gas, max. number of electrons, and the last line shows the values n+ l. There are, however, deviations from this filling procedure.
Between stationary states Quantum transitions are possible in an atom. When moving from more high level energy E i to a lower E k the atom gives up energy (E i - E k), and during the reverse transition receives it. During radiative transitions, an atom emits or absorbs a quantum of electromagnetic radiation (photon). Non-radiative transitions are also possible, when an atom gives or receives energy when interacting with other particles that it collides with (for example, in gases) or is associated for a long time (in molecules, liquids and solids). In atomic gases, as a result of a collision of a free atom with another particle, it can move to another energy level - experience an inelastic collision; during an elastic collision, only the kinetic energy of the translational motion of the atom changes, and its total internal energy E remains unchanged. An inelastic collision of a free atom with a rapidly moving electron, which transfers its kinetic energy to this atom - excitation of the atom by electron impact - is one of the methods for determining the energy levels of an atom.
Structure and properties of substances. Chemical properties are determined by the structure of the outer electron shells of the atom, in which electrons are bound relatively weakly (binding energies from several eV to several tens of eV). The structure of the outer shells of atoms of chemical elements of one group (or subgroup) is periodic. systems are similar, which determines the similarity chemical properties these elements. As the number of electrons in a filling shell increases, their binding energy, as a rule, increases; Electrons in a closed shell have the highest binding energy. Therefore, atoms with one or more electrons in a partially filled outer shell donate them to chemical reactions. Atoms that lack one or more to form a closed outer shell usually accept them. Noble gas atoms, which have closed outer shells, do not enter into chemical reactions under normal conditions.
Structure inner shells atoms whose electrons are bound much more tightly (binding energy 10 2 -10 4 eV), manifests itself only when atoms interact with fast particles and high-energy photons. Such interactions determine the nature of X-ray spectra and the scattering of particles (electrons, neutrons) on atoms. The mass of an atom determines its physical properties such as momentum and kinetic energy. Some subtle physical effects (NMR, NQR, hyperfine structure of spectral lines) depend on the mechanical and associated magnetic and electrical moments of the atomic nucleus.
Weaker compared to chemical bonds electrostatic interactions two atoms are manifested in their mutual polarizability - displacement relative to the nuclei and the emergence of polarization forces of attraction between atoms. The atom is also polarized in external electric fields; As a result, the energy levels are shifted and, most importantly, the degenerate levels are split. An atom can also be polarized under the influence of an electric field, a wave of electromagnetic radiation; polarization depends on the frequency of radiation, which determines the dependence of the refractive index of the substance associated with the polarizability of the atom on it. Close connection optical properties atom with its electrical properties are especially pronounced in the optical spectra.
The outer electrons of an atom determine and magnetic properties substances. In an atom with filled outer shells, its magnetic moment, like the total angular momentum (mechanical moment), equal to zero. Atoms with partially filled outer shells have, as a rule, permanent magnetic moments that are different from zero; such substances are paramagnetic. In an external magnetic field, all atomic energy levels for which the magnetic moment is not zero are split. All atoms have diamagnetism, which is caused by the appearance of an induced magnetic moment in them under the influence of an external magnetic field.
The properties of an atom in a bound state (for example, part of a molecule) differ from the properties of free atoms; the properties determined by outer electrons, taking part in chemical bond; the properties determined by the electrons of the inner shells may remain practically unchanged. Some properties of an atom may undergo changes depending on the symmetry of the environment of a given atom. An example is the splitting of atomic energy levels in crystals and complex compounds, which occurs under the influence of electric fields created by surrounding or ligands.
Literature: Karapetyants M. X., Drakin S. I., Structure of matter, 3rd ed., M., 1978; Shlolevsky E.V., Atomic physics, 7th ed., vol. 1-2, M., 1984. M.A. Elyashevich.
Select the first letter in the article title:
Atom(from ancient Greek ἄτομος - indivisible) - a particle of a substance of microscopic size and mass, the smallest part of a chemical element, which is the bearer of its properties.An atom is made up of atomic nucleus and electrons. If the number of protons in the nucleus coincides with the number of electrons, then the atom as a whole turns out to be electrically neutral. Otherwise, it has some positive or negative charge and is called an ion. In some cases, atoms are understood only as electrically neutral systems in which the charge of the nucleus is equal to the total charge of the electrons, thereby contrasting them with electrically charged ions.
Core, which carries almost the entire (more than 99.9%) of the mass of an atom, consists of positively charged protons and uncharged neutrons bound together through strong interaction. Atoms are classified according to the number of protons and neutrons in the nucleus: the number of protons Z corresponds to the serial number of the atom in the periodic table and determines its belonging to a certain chemical element, and the number of neutrons N - a specific isotope of this element. The Z number also determines the total positive electric charge(Ze) of the atomic nucleus and the number of electrons in a neutral atom, which determines its size.
Atoms various types in different quantities, connected by interatomic bonds, form molecules.
Properties of the atom
By definition, any two atoms with the same number of protons in their nuclei belong to the same chemical element. Atoms with the same number of protons but different numbers of neutrons are called isotopes of a given element. For example, hydrogen atoms always contain one proton, but there are isotopes without neutrons (hydrogen-1, sometimes also called protium - the most common form), with one neutron (deuterium) and two neutrons (tritium). The known elements form a continuous natural series according to the number of protons in the nucleus, starting with the hydrogen atom with one proton and ending with the ununoctium atom, which has 118 protons in the nucleus. All isotopes of the elements of the periodic table, starting with number 83 (bismuth), are radioactive.
Weight
Since protons and neutrons make the largest contribution to the mass of an atom, the total number of these particles is called the mass number. The rest mass of an atom is often expressed in atomic mass units (a.m.u.), which is also called a dalton (Da). This unit is defined as 1⁄12th of the rest mass of a neutral carbon-12 atom, which is approximately equal to 1.66 × 10−24 g. Hydrogen-1 is the lightest isotope of hydrogen and the atom with the smallest mass, having an atomic weight of about 1.007825 a. e.m. The mass of an atom is approximately equal to the product of the mass number per atomic mass unit. The heaviest stable isotope is lead-208 with a mass of 207.9766521 a. eat.
Since the masses of even the heaviest atoms in ordinary units (for example, grams) are very small, moles are used in chemistry to measure these masses. One mole of any substance, by definition, contains the same number of atoms (approximately 6.022·1023). This number (Avogadro's number) is chosen in such a way that if the mass of an element is 1 a. e.m., then a mole of atoms of this element will have a mass of 1 g. For example, carbon has a mass of 12 a. e.m., so 1 mole of carbon weighs 12 g.
Size
Atoms do not have a clearly defined external boundary, so their sizes are determined by the distance between the nuclei of neighboring atoms that have formed a chemical bond (Covalent radius) or by the distance to the farthest stable electron orbit in the electron shell of this atom (Atomic radius). The radius depends on the position of the atom in the periodic table, the type of chemical bond, the number of nearby atoms (coordination number) and a quantum mechanical property known as spin. In the periodic table of elements, the size of an atom increases as you move down a column and decreases as you move down a row from left to right. Accordingly, the smallest atom is a helium atom with a radius of 32 pm, and the largest is a cesium atom (225 pm). These sizes are thousands of times smaller than the wavelength of visible light (400-700 nm), so atoms cannot be seen with an optical microscope. However, individual atoms can be observed using a scanning tunneling microscope.
The smallness of atoms is demonstrated by the following examples. A human hair is a million times thicker than a carbon atom. One drop of water contains 2 sextillion (2 1021) oxygen atoms, and twice as many hydrogen atoms. One carat of diamond weighing 0.2 g consists of 10 sextillion carbon atoms. If an apple could be enlarged to the size of the Earth, then the atoms would reach the original size of the apple.
Scientists from the Kharkov Institute of Physics and Technology presented the first photographs of an atom in the history of science. To obtain images, scientists used an electron microscope that records radiation and fields (field-emission electron microscope, FEEM). Physicists sequentially placed dozens of carbon atoms in a vacuum chamber and passed an electrical discharge of 425 volts through them. The radiation of the last atom in the chain onto a phosphorus screen made it possible to obtain an image of a cloud of electrons around the nucleus.
Molecule(novolat. molecule, abbreviated from Latin. moles-mass), a microparticle formed from two or more atoms and capable of independent existence. It has a constant composition (qualitative and quantitative) of the atomic nuclei included in it and a fixed number of electrons and has a set of properties that make it possible to distinguish one molecule from others, including from molecules of the same composition. A molecule, as a system consisting of interacting electrons and nuclei, can be in different states and move from one state to another forcedly (under the influence of external influences) or spontaneously. All molecules of a given type are characterized by a certain set of states, which can serve to identify the molecules. As an independent formation, a molecule has in each state a certain set of physical properties; these properties are preserved to one degree or another during the transition from molecules to the substance consisting of them and determine the properties of this substance. During chemical transformations, molecules of one substance exchange atoms with molecules of another substance, break up into molecules with fewer atoms, and also enter into other types of chemical reactions. Therefore, chemistry studies substances and their transformations in inextricable connection with the structure and state of molecules
Typically, a molecule is an electrically neutral particle; if a molecule carries an electrical charge (positive or negative), then we speak of molecular ions (cations or anions, respectively). In a substance, positive ions always coexist with negative ones. Molecules that are in states with a multiplicity different from unity (usually in doublet states) are called radicals. Free radicals under normal conditions, as a rule, cannot exist for a long time. However, free radicals of a relatively complex structure are known, which are quite stable and can exist under normal conditions.
Based on the number of atomic nuclei included in the molecule, molecules are distinguished as diatomic, triatomic, etc. If the number of atoms in a molecule exceeds hundreds and thousands, the molecule is called a macromolecule. The sum of the masses of all the atoms that make up the molecule is considered as the molecular mass (see also Molecular mass of a polymer. Molecular mass distribution). By size molecular weight All substances are conventionally divided into low- and high-molecular.
Atom(from ancient Greek ἄτομος - indivisible) - a particle of a substance of microscopic size and mass, the smallest part of a chemical element, which is the bearer of its properties.
The idea of atoms as indivisible smallest particles of matter arose in ancient times, but only in the 18th century, through the works of A. Lavoisier, M.V. Lomonosov and other scientists, the reality of the existence of atoms was proven.
General characteristics of the structure of the atom. An atom consists of a positively charged nucleus surrounded by a cloud of negatively charged electrons. The dimensions of an atom as a whole are determined by the dimensions of its electron cloud and are large compared to the dimensions of the atomic nucleus (the linear dimensions of an atom are ~ 10~8 cm, its nucleus ~ 10" -10" 13 cm). The electron cloud of an atom does not have strictly defined boundaries, so the size of the atom means. the degrees are arbitrary and depend on the methods of their determination (see Atomic radii). The nucleus of an atom consists of Z protons and N neutrons held together by nuclear forces (see Atomic nucleus). Positive proton charge and negative. the charge of the electron is the same in absolute terms. magnitude and are equal to e = 1.60*10 -19 C; the neutron does not have electricity. charge. Nuclear charge +Ze - basic. characteristic of an atom that determines its belonging to a particular chemical. element. The serial number of the element in the periodic period. periodic system (atomic number) is equal to the number of protons in the nucleus.
In an electrically neutral atom, the number of electrons in the cloud is equal to the number of protons in the nucleus. However, under certain conditions, it can lose or gain electrons, turning, respectively. in positive or deny. ion, e.g. Li + , Li 2+ or O - , O 2- . When talking about atoms of a certain element, we mean both neutral atoms and ions of this element.
Atomic structure and propertiessubstances. Chem. Saints are determined by the structure of the exterior. electron shells of atoms, in which the electrons are bonded relatively weakly (binding energies from several eV to several tens of eV). External structure shells of chemical atoms. elements of one group (or subgroup) periodic. systems are similar, which determines the similarity of chemicals. St. in these elements. (1) With an increase in the number of electrons in the filling shell, their binding energy, as a rule, increases; max. electrons in a closed shell have binding energy. Therefore, atoms with one or more. electrons in a partially filled ext. the shell is given to the chemical. r-tions. Atoms, Crimea is missing one or more. electrons for the formation of a closed external. shells usually accept them. Atoms of noble gases with closed external shells, under normal conditions do not enter into chemical reactions. districts.
Internal structure shells of atoms, the electrons of which are bound much more tightly (binding energy 10 2 -10 4 eV), appears only during interaction. atoms with fast particles and high-energy photons. Such interactions determine the nature of X-ray spectra and the scattering of particles (electrons, neutrons) on atoms (see Diffraction methods). The mass of an atom determines its physical properties. holy, like an impulse, kinetic. energy. From mechanical and related mag. and electric moments of the atomic nucleus depend on certain subtle physical factors. effects (NMR, NQR, hyperfine structure of spectral lines, see Spectroscopy).
1 footnote: Electron-volt(rarely electronvolt; Russian designation: eV, international: eV) - an extra-system unit of energy used in atomic and nuclear physics, in elementary particle physics and in close and related fields of science (biophysics, physical chemistry, astrophysics, etc.). In the Russian Federation, the electron volt is approved for use as an off-system unit without any limitation on the period of application.
Nuclear model of the atom
At the beginning of the 20th century, as a result of the study of cathode rays, negative particles were discovered - electrons with a charge of 1.6. 10‾ 19 C, mass 9.11. 10‾ 31 kg, open x-ray electromagnetic radiation. Having summarized these discoveries, J. Thomson in 1897 proposed his model of the atom - it is a positively charged sphere in which negative electrons are interspersed (like raisins in a pudding). If this model is correct, then the metal foil is a film of positive electricity containing electrons and the flow of α-particles should easily penetrate through it without changing direction.
In 1909, employees of the English. scientist E. Rutherford checked this. 1 out of 100,000 α-particles, when passing through gold foil, were scattered at large angles and even turned back. Analyzing the results of the experiment, Rutherford concluded that the mass and charge of the atom are concentrated in a small part of the volume called the nucleus. Those α-particles that collide with nuclei are rejected. Most α particles pass through the space between the nuclei. The model of atomic structure proposed by E. Rutherford resembled the solar system. It is called the planetary model. According to it, at the center of the atom there is a positive nucleus in which the entire mass of the atom is concentrated. Electrons move around the nucleus in circular orbits. The charge of the nucleus and the number of electrons are the same, i.e. atom is a neutral particle.
In 1913 English physicist Moseley measured the wavelengths of X-rays emitted by different metals in a cathode tube and plotted the dependence of the inverse square root of the X-ray wavelength on the atomic number of the element. This graph (Fig. 1) shows that the serial number reflects some important characteristic of the element. Moseley proposed that this characteristic was the charge on the nucleus of an atom, and that it increased by one when moving from one element to the next in order. He called the atomic number the atomic number - Z.
Moseley's Law:
The square root of the reciprocal of the wavelength of X-rays emitted by atoms of various elements is found in linear dependence from the element's serial number.
This is a law that relates the frequency of the spectral lines of the characteristic X-ray radiation of an atom of a chemical element with its atomic number.
where is the wavelength, A– constant value, Z– serial number of the element (nuclear charge).
Later it became known that the atomic number is equal to the number of protons in the nucleus. Thus, the atomic number is equal to the charge of the nucleus and it also determines the presence of protons (positive particles) in it. And since atoms are neutral, the number of electrons in an atom must be equal to the number of protons. But the masses of the atoms turned out to be greater than the total mass of protons. To explain the excess mass, the existence of neutrons was suggested. These particles should have the same mass as a proton, but zero charge (1.675 - 10 - 27 kg). The neutron was discovered by Rutherford's collaborator Chadwig in 1932. It was finally established that the atom consists of a nucleus and electrons, and the nucleus of protons and neutrons. Their sum is called nucleon number or massive - A.
A= Z+ N,
Z- number of protons, N- number of neutrons.
Atoms with different numbers of protons ( Z) and neutrons ( N), but with the same number of nucleons A, called isobars . For example,
Isotopes – atoms with the same number of protons ( Z), nose different numbers nucleons
Isotones – atoms with the same number of neutrons ( N)
Thus, fractional values of atomic masses in the periodic table are explained by the presence of isotopes for the same element.
Atomic nucleus- the central part of the atom, in which the bulk of its mass is concentrated (more than 99.9%). The nucleus is positively charged; the charge of the nucleus is determined by the chemical element to which the atom belongs. The dimensions of the nuclei of various atoms are several femtometers, which is more than 10 thousand times smaller than the size of the atom itself.
Spectral line- a feature of a part of the spectrum, expressed in a local increase (light, emission lines, spectral maxima) or decrease (dark lines, absorption lines, spectral minima) of the signal level.
Residual intensity called the enhancement/attenuation of radiation in a spectral line compared to a continuous spectrum.
The function characterizing the dependence of the residual intensity on frequency is called the line profile.
X-ray radiation- electromagnetic waves, the energy of photons of which lies on the scale of electromagnetic waves between ultraviolet radiation and gamma radiation, which corresponds to wavelengths from 10 −2 to 10 2 Å (from 10 −12 to 10 −8 m).
Photon(from ancient Greek φῶς, native pad. φωτός, “light”) - an elementary particle, a quantum of electromagnetic radiation (in the narrow sense of light). It is a massless particle, capable of existing in a vacuum only by moving at the speed of light.
