In this article you will learn about the 10 most everyday chemical reactions in life!
Reaction No. 1 - Photosynthesis
Plants use a chemical reaction. photosynthesisto convert carbon dioxide to water, food and oxygen. Photosynthesis - one of the most common and important chemical reactions in life. Only through photosynthesis do plants produce food for themselves and animals, does it turn carbon dioxide into oxygen. 6 CO2 + 6 H2O + light → C6H12O6 + 6 O2
 Reaction No. 2 - Aerobic cellular respiration
Reaction No. 2 - Aerobic cellular respiration
Aerobic cell respiration - This is the opposite process of photosynthesis in that the energy of the molecules in combination with the oxygen we breathe in order to release the energy needed by our cells, plus carbon dioxide and water. The energy used by cells is a chemical reaction in ATP format.
The general equation for aerobic cell respiration: C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATPs)
Reaction No. 3 - Anaerobic respiration
Unlike aerobic cell respiration, anaerobic respiration describes a set of chemical reactions that allow cells to receive energy from complex molecules without oxygen. Your cells in the muscles perform anaerobic respiration when you run out of oxygen supplied by them, for example, during intense or prolonged exercise. Anaerobic respiration of yeast and bacteria is used for fermentation, production of ethanol, carbon dioxide and other chemicals that produce cheese, wine, beer, bread and many other food products.
General chemical equation for anaerobic respiration: C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Reaction No. 4 - Combustion
Each time you light a match, light a candle, light a fire or light a grill, you see a burning reaction. Combustion reaction combines energy molecules with oxygen to form carbon dioxide and water.
For example, the propane combustion reaction found in gas grills and some fireplaces is: C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
 Reaction No. 5 - Rust
Reaction No. 5 - Rust
Over time, the iron turns red, a puff cover called rust. This is an example of an oxidation reaction. Other household items include the formation of a yar-copperhead.
The chemical equation for rust iron: Fe + O 2 + H 2 O → Fe 2 O 3. XH 2 O
 Reaction No. 6 - Mixing chemicals
Reaction No. 6 - Mixing chemicals
If you mix vinegar with baking soda or milk with a baking powder in the recipe, you will see how the exchange of reactions will occur. The ingredients recombine to form carbon dioxide and water. Carbon dioxide forms bubbles and helps baking rise.
In practice, this reaction is quite simple, but often consists of several stages. Here is the general chemical equation for the reaction of soda with vinegar: HC 2 H 3 O 2 (aq) + NaHCO 3 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O () + CO 2 (g)
 Reaction No. 7 - Battery
Reaction No. 7 - Battery
Electrochemical or redox reactions batteries used to convert chemical energy into electrical energy. Spontaneous redox reactions occur in galvanic cells, while spontaneous redox reactions occur in electrolyzers.

Reaction No. 8 - Digestion
Thousands of chemical reactions occur in the process. digestion. Once you put food in your mouth, an enzyme in your saliva, amylasebegins to break down sugar and other hydrocarbons into simpler forms so that you can absorb food. Hydrochloric acid in the stomach, it reacts with food to break it down, while enzymes break down proteins and fats so that they can pass through the blood through the intestinal walls.
 Reaction No. 9 - Acid-alkaline
Reaction No. 9 - Acid-alkaline
Whenever you combine acids with a base, you perform acid-base reaction. This reaction is the neutralization of acid and base with the formation of salt and water.
Chemical equation for acid-base reactionwhich produces potassium chloride: HCl + KOH → KCl + H 2 O
 Reaction No. 10 - Soap and detergents
Reaction No. 10 - Soap and detergents
Soaps and detergents are obtained by pure chemical reactions. Soap turns dirt into an emulsion, which means oil stains are bound to soap so they can be removed with water. Detergents act as surfactants, lowering the surface tension of water so that they can interact with oils, isolate and rinse them.
12. PHYSICAL AND CHEMICAL PHENOMENA.
EQUATIONS OF CHEMICAL REACTIONS.
From the course of natural science and physics, you know that with bodies and substances there are changes that are divided into physical and chemical
Any chemical reaction is accompanied by external signs, by which we judge its course. It:
1. The appearance of sediment.
2.Color change.
3. The evolution of gas.
4. Absorption or release of heat.
For a chemical reaction to occur, several conditions are necessary. The first is bringing into contact of reacting substances; the second is the grinding of substances (the greatest grinding is achieved by dissolving the substances); third, for many reactions to occur, it is necessary to heat the reacting substances to a certain temperature.
Chemical reactions can be expressed in writing, using the equations of chemical reactions, which are often called chemical equations. What is it?
A chemical equation is a conditional record of a chemical reaction using chemical formulas and coefficients.
When compiling the equations of reactions, it is necessary to use the law of conservation of the mass of substances discovered by M.V. Lomonosov and A. Lavoisier. The mass of substances that have entered into a reaction is equal to the mass of substances resulting from it. But you know that substances are composed of atoms, so when drawing up chemical equations, we will use the rule: the number of atoms of each chemical element of the starting materials should be equal to the number of atoms in the reaction products.Algorithm for compiling reaction equations.
Consider the algorithm for compiling chemical equations using the example of the interaction of simple substances: metals and non-metals with each other. Let phosphorus and oxygen interact (combustion reaction).
1. Write down these substances next to each other, put a “+” sign between them (here we will take into account that oxygen is a diatomic molecule), and after them an arrow is like an equal sign.
P + O 2
2. We write down the reaction product formula after the arrow:
P + O 2 P 2 O 5
3. It can be seen from the diagram that on the left there are oxygen-2 atoms, on the right-5, and in accordance with the law of conservation of mass of substances, the number of atoms of a given chemical element must be the same. To equalize their number, we find the least common multiple. For 2 and 5 it will be the number 10. Divide the least common multiple by the number of atoms in the formulas. 10: 2 \u003d 5, 10: 5 \u003d 2, these will be the coefficients that are placed before oxygen O 2 and phosphorus oxide (V) P 2 O 5, respectively.
P + 5O 2 2P 2 O 5
oxygen left and right became 10 (5 · 2 \u003d 10, 2 · 5 \u003d 10)4. The coefficient refers to the whole formula and is placed in front of it. After setting it to the right, phosphorus became 2 · 2 \u003d 4 atoms. And on the left is 1 (coefficient 1 is not set). So we put coefficient 4 in front of phosphorus.
4P + 5O 2 2P 2 O 5
This is the final record of the chemical equation.
It reads: four ne plus five o-two equals two ne-two o-five.
Physical they call such phenomena in which there is no transformation of some substances into others, but their aggregate states, shape and size of bodies change.
Examples: ice melting, wire drawing, granite crushing, water evaporation.
Chemical call such phenomena in which there is a transformation of some substances into others.
Examples: burning wood, blackening of copper, rusting of iron.
In the future, we will call chemical phenomena chemical reactions.
Signs of a chemical reaction. They can be used to judge whether a chemical reaction between reactants has passed or not. These signs are usually attributed to the following:
· Color change: CuSO4 (cyan) \u003d Cu 2+ + SO4 2-
· Precipitation: CO 2 + Ca (OH) 2 CaCO 3 + H 2 O
· Gas evolution: CaCO 3 + HCl CaCl 2 + CO 2 + H 2 O
· The formation of slightly dissociated substances: 2NaOH + H 2 SO 4 \u003d Na 2 SO 4 + 2H 2 O
· Energy release (thermal or light): 2 H 2 (g) + O 2 (g) \u003d 2 H 2 O (g) + 572 kJ
1. Close contact of the reacting substances (necessary): H 2 SO 4 + Zn \u003d ZnSO 4 + H 2 2. Heating (possibly) a) to start the reaction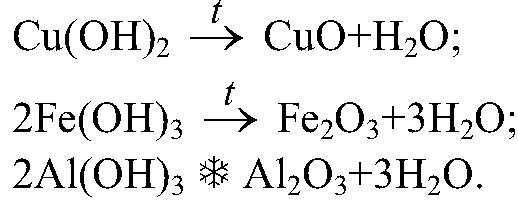 b) constantly Classification of chemical reactions according to various criteria 1.By the presence of a phase boundary, all chemical reactions are divided into homogeneous and heterogeneous A chemical reaction occurring within one phase is called homogeneous chemical reaction. A chemical reaction occurring at the phase boundary is called heterogeneous chemical reaction. In a multi-stage chemical reaction, some stages may be homogeneous, while others may be heterogeneous. Such reactions are called homogeneous heterogeneous . Depending on the number of phases that form the starting materials and reaction products, chemical processes can be homophasic (starting materials and products are within the same phase) and heterophasic (starting materials and products form several phases). The homo- and heterophasicity of the reaction is not related to whether the reaction is homo- or heterogeneous. Therefore, four types of processes can be distinguished: Homogeneous reactions (homophasic). In reactions of this type, the reaction mixture is homogeneous, and the reactants and products belong to the same phase. An example of such reactions are ion exchange reactions, for example, neutralization of an acid solution with an alkali solution: Heterogeneous homophase reactions. The components are within the same phase, however, the reaction proceeds at the phase boundary, for example, on the surface of the catalyst. An example would be the hydrogenation of ethylene on a nickel catalyst: Homogeneous heterophase reactions. Reagents and products in such a reaction exist within several phases, however, the reaction proceeds in one phase. Thus, the oxidation of hydrocarbons in the liquid phase with gaseous oxygen can take place. Heterogeneous heterophasic reactions. In this case, the reagents are in a different phase state, the reaction products can also be in any phase state. The reaction process proceeds at the phase boundary. An example is the reaction of salts of carbonic acid (carbonates) with Bronsted acids: 2. By changing the degrees of oxidation of reagents [edit | edit wiki text] In this case, there are Redox reactions in which atoms of one element (oxidizer) are recovering
, that is, they lower their oxidation state, and the atoms of another element (reducing agent) oxidized
, that is, increase their oxidation state. A particular case of redox reactions is the reaction of disproportionation, in which the oxidizing agent and reducing agent are atoms of the same element, which are in different degrees of oxidation. An example of a redox reaction is the combustion of hydrogen (a reducing agent) in oxygen (an oxidizing agent) to form water: An example of a disproportionation reaction is a decomposition reaction of ammonium nitrate when heated. In this case, the oxidizing agent is nitrogen (+5) nitro groups, and the reducing agent is nitrogen (-3) of the ammonium cation: Do not apply to redox reactions in which there is no change in the degree of oxidation of atoms, for example: 3. By the thermal effect of the reaction All chemical reactions are accompanied by the release or absorption of energy. When chemical bonds are broken in the reagents, energy is released, which mainly goes to the formation of new chemical bonds. In some reactions, the energies of these processes are close, and in this case, the overall thermal effect of the reaction approaches zero. In other cases, we can distinguish: exothermic reactions that occur with the release of heat (positive thermal effect) CH 4 + 2О 2 \u003d СО 2 + 2Н 2 О + energy (light, heat); CaO + H 2 O \u003d Ca (OH) 2 + energy (heat). endothermic reactions during which heat is absorbed (negative thermal effect) from the environment. Ca (OH) 2 + energy (heat) \u003d CaO + H 2 O The thermal effect of the reaction (reaction enthalpy, Δ r H), which is often very important, can be calculated according to Hess's law if the enthalpies of formation of reactants and products are known. When the sum of the enthalpies of the products is less than the sum of the enthalpies of the reactants (Δ r H< 0) наблюдается выделение тепла, в противном случае (Δ r H > 0) is the absorption. 4. By the type of transformation of reacting particles [edit | edit wiki text] compounds: decompositions: substitutions: exchange (including the type of reaction — neutralization): Chemical reactions are always accompanied by physical effects: absorption or release of energy, color change of the reaction mixture, etc. These physical effects are often judged about the course of chemical reactions. Compound Reaction-chemical reaction, as a result of which two or more starting materials form only one new one. Both simple and complex substances can enter into such reactions. Decomposition reaction-chemical reaction, as a result of which several new substances are formed from one substance. Only complex compounds enter into reactions of this type, and both complex and simple substances can be their products. Substitution reaction-chemical reaction, as a result of which the atoms of one element, which are part of a simple substance, replace the atoms of another element in its complex compound. As follows from the definition, in such reactions one of the starting materials should be simple and the other complex. Exchange reactions- a reaction as a result of which two complex substances exchange their constituent parts 5. According to the direction of flow, chemical reactions are divided into irreversible and reversible Irreversible
b) constantly Classification of chemical reactions according to various criteria 1.By the presence of a phase boundary, all chemical reactions are divided into homogeneous and heterogeneous A chemical reaction occurring within one phase is called homogeneous chemical reaction. A chemical reaction occurring at the phase boundary is called heterogeneous chemical reaction. In a multi-stage chemical reaction, some stages may be homogeneous, while others may be heterogeneous. Such reactions are called homogeneous heterogeneous . Depending on the number of phases that form the starting materials and reaction products, chemical processes can be homophasic (starting materials and products are within the same phase) and heterophasic (starting materials and products form several phases). The homo- and heterophasicity of the reaction is not related to whether the reaction is homo- or heterogeneous. Therefore, four types of processes can be distinguished: Homogeneous reactions (homophasic). In reactions of this type, the reaction mixture is homogeneous, and the reactants and products belong to the same phase. An example of such reactions are ion exchange reactions, for example, neutralization of an acid solution with an alkali solution: Heterogeneous homophase reactions. The components are within the same phase, however, the reaction proceeds at the phase boundary, for example, on the surface of the catalyst. An example would be the hydrogenation of ethylene on a nickel catalyst: Homogeneous heterophase reactions. Reagents and products in such a reaction exist within several phases, however, the reaction proceeds in one phase. Thus, the oxidation of hydrocarbons in the liquid phase with gaseous oxygen can take place. Heterogeneous heterophasic reactions. In this case, the reagents are in a different phase state, the reaction products can also be in any phase state. The reaction process proceeds at the phase boundary. An example is the reaction of salts of carbonic acid (carbonates) with Bronsted acids: 2. By changing the degrees of oxidation of reagents [edit | edit wiki text] In this case, there are Redox reactions in which atoms of one element (oxidizer) are recovering
, that is, they lower their oxidation state, and the atoms of another element (reducing agent) oxidized
, that is, increase their oxidation state. A particular case of redox reactions is the reaction of disproportionation, in which the oxidizing agent and reducing agent are atoms of the same element, which are in different degrees of oxidation. An example of a redox reaction is the combustion of hydrogen (a reducing agent) in oxygen (an oxidizing agent) to form water: An example of a disproportionation reaction is a decomposition reaction of ammonium nitrate when heated. In this case, the oxidizing agent is nitrogen (+5) nitro groups, and the reducing agent is nitrogen (-3) of the ammonium cation: Do not apply to redox reactions in which there is no change in the degree of oxidation of atoms, for example: 3. By the thermal effect of the reaction All chemical reactions are accompanied by the release or absorption of energy. When chemical bonds are broken in the reagents, energy is released, which mainly goes to the formation of new chemical bonds. In some reactions, the energies of these processes are close, and in this case, the overall thermal effect of the reaction approaches zero. In other cases, we can distinguish: exothermic reactions that occur with the release of heat (positive thermal effect) CH 4 + 2О 2 \u003d СО 2 + 2Н 2 О + energy (light, heat); CaO + H 2 O \u003d Ca (OH) 2 + energy (heat). endothermic reactions during which heat is absorbed (negative thermal effect) from the environment. Ca (OH) 2 + energy (heat) \u003d CaO + H 2 O The thermal effect of the reaction (reaction enthalpy, Δ r H), which is often very important, can be calculated according to Hess's law if the enthalpies of formation of reactants and products are known. When the sum of the enthalpies of the products is less than the sum of the enthalpies of the reactants (Δ r H< 0) наблюдается выделение тепла, в противном случае (Δ r H > 0) is the absorption. 4. By the type of transformation of reacting particles [edit | edit wiki text] compounds: decompositions: substitutions: exchange (including the type of reaction — neutralization): Chemical reactions are always accompanied by physical effects: absorption or release of energy, color change of the reaction mixture, etc. These physical effects are often judged about the course of chemical reactions. Compound Reaction-chemical reaction, as a result of which two or more starting materials form only one new one. Both simple and complex substances can enter into such reactions. Decomposition reaction-chemical reaction, as a result of which several new substances are formed from one substance. Only complex compounds enter into reactions of this type, and both complex and simple substances can be their products. Substitution reaction-chemical reaction, as a result of which the atoms of one element, which are part of a simple substance, replace the atoms of another element in its complex compound. As follows from the definition, in such reactions one of the starting materials should be simple and the other complex. Exchange reactions- a reaction as a result of which two complex substances exchange their constituent parts 5. According to the direction of flow, chemical reactions are divided into irreversible and reversible Irreversible 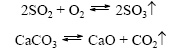 chemical reactions that occur in only one direction (" from left to right"), as a result of which the starting materials are converted into reaction products. They say about such chemical processes that they proceed" to the end. " combustion reactions, as well as reactions accompanied by the formation of sparingly soluble or gaseous substances Reversible
chemical reactions that occur in only one direction (" from left to right"), as a result of which the starting materials are converted into reaction products. They say about such chemical processes that they proceed" to the end. " combustion reactions, as well as reactions accompanied by the formation of sparingly soluble or gaseous substances Reversible 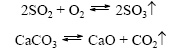 chemical reactions that occur simultaneously in two opposite directions (“left to right” and “right to left”) are called. In the equations of such reactions, the equal sign is replaced by two oppositely directed arrows. Among two simultaneously occurring reactions, direct (flows "from left to right") and the opposite(proceeds from “right to left”). Since, during a reversible reaction, the starting materials are simultaneously consumed and formed, they do not completely turn into reaction products. Therefore, it is said that reversible reactions do not proceed to the end. The result is always a mixture of starting materials and reaction products. 6. Based on the participation of the catalysts, chemical reactions are divided into catalytic and non-catalytic Catalytic 2SO 2 + O 2 → 2SO 3 (V 2 O 5 catalyst) is defined as reactions occurring in the presence of catalysts. In the equations of such reactions, the chemical formula of the catalyst is indicated with an equal or reversible sign, sometimes together with a flow condition. Reactions of this type include many decomposition reactions and compounds. Non-catalytic 2NO + O2 \u003d 2NO 2 refers to many reactions that occur in the absence of catalysts, such as, for example, exchange and substitution reactions.
chemical reactions that occur simultaneously in two opposite directions (“left to right” and “right to left”) are called. In the equations of such reactions, the equal sign is replaced by two oppositely directed arrows. Among two simultaneously occurring reactions, direct (flows "from left to right") and the opposite(proceeds from “right to left”). Since, during a reversible reaction, the starting materials are simultaneously consumed and formed, they do not completely turn into reaction products. Therefore, it is said that reversible reactions do not proceed to the end. The result is always a mixture of starting materials and reaction products. 6. Based on the participation of the catalysts, chemical reactions are divided into catalytic and non-catalytic Catalytic 2SO 2 + O 2 → 2SO 3 (V 2 O 5 catalyst) is defined as reactions occurring in the presence of catalysts. In the equations of such reactions, the chemical formula of the catalyst is indicated with an equal or reversible sign, sometimes together with a flow condition. Reactions of this type include many decomposition reactions and compounds. Non-catalytic 2NO + O2 \u003d 2NO 2 refers to many reactions that occur in the absence of catalysts, such as, for example, exchange and substitution reactions. 3 Question 3
The development of ideas about the structure of matter:
Chemical reactions in our daily lives Project participants: 1. Savostyanova Evgenia Konstantinovna grade 9 2. Zadorina Elizaveta Vadimovna grade 8 3. Ermakov Pavel Igorevich grade 9 4. Dmitriev Ilya Alekseevich grade 9 5. Katasonov Nikita Sergeevich grade 9 Head: Lazareva Elena Alexandrovna 2014 year Municipal budgetary educational institution "Secondary school 17"
The relevance of the chosen topic Nowadays, millions of different substances are known. Many of them are used not only in industry and agriculture, but also in everyday life. Unfortunately, not all people have basic chemical knowledge about substances and their transformations. We believe that even from school, it is necessary to instill chemical literacy. Therefore, the topic "Chemical reactions in our daily lives" will be relevant.


Natural gas combustion Russia is a leader in natural gas reserves and production. Therefore, in our homes we use the combustion reaction of natural gas to produce thermal energy. Natural gas is a mixture of gases formed in the bowels of the Earth during anaerobic decomposition of organic substances. Chemical composition: ethane (C 2 H 6), propane (C 3 H 8) butane (C 4 H 10). As well as other non-hydrocarbon substances: hydrogen (H 2), hydrogen sulfide (H 2 S), carbon dioxide (CO 2), nitrogen (N 2), helium (He). The bulk of natural gas is methane (CH 4) from 92 to 98%. It is a colorless, light, flammable gas, odorless, almost insoluble in water. A mixture of methane in the air is explosive. Methane combustion reaction CH 4 + 2O 2 \u003d CO 2 + 2H 2 O + Q. Methane burns with a bluish or almost colorless flame, releasing a large amount of heat (879 kJ / mol). When using gas equipment in a house, it is necessary: \u200b\u200bto check the chimney, ventilate the room, monitor the state of gas pipelines, and not leave working gas equipment unattended.

Burning matches With a large selection of various lighters, matches are very popular. What processes occur during the ignition of a match? Here she was struck about the boxes. There was a flame and a pungent smell of "sulfur." The process began under the action of friction. First, red phosphorus caught fire, which was on a matchbox 4Р + 5О 2 \u003d 2Р 2 О 5 Phosphorus, which gives high friction during friction, set fire to a mixture of sulfur and bertholite salt in a match head S + O 2 \u003d SO 2 (SO 2 - sulfur dioxide, source of pungent odor). The head set fire to wood С 6 Н 10 О 5 + 6О 2 \u003d 6СО 2 + 5Н 2 О Almost all combustion products are harmful to the body. Only when one match is burned, an insignificant amount of them is released, which does not have a significant effect on a person. But when using matches, a chemically educated person should remember that “MATCHES ARE NOT A LOAD!”

Hydrolysis of soap In production and everyday life, soap is the name for technical mixtures of water-soluble salts of higher fatty acids, often with the addition of some other substances that have a detergent effect. The basis of the mixtures is usually made up of sodium (less often potassium and ammonium) salts of saturated and unsaturated fatty acids with the number of carbon atoms in the molecule from 12 to 18 (stearic, palmitic, myristic, lauric and oleic). Soaps often also include salts of naphthenic and resin acids, and sometimes other compounds with detergent in solutions. Soaps are formed by a strong base and weak acid, therefore, they are easily hydrolyzed: C 17 H 35 COONa + H 2 O \u003d C 17 H 35 COOH + NaOH The alkaline hydrolysis medium, therefore, soaps are quite aggressive to the skin and their frequent use degreases . There are a great many varieties and brands of soap, and before choosing the most suitable, you need to determine the type of your skin. Oily skin often shines due to strong sweat and greasy separation, usually large pores on it. 2 hours after washing, oily skin stains on a napkin applied to the face. Such skin requires a soap with a mild draining effect. Dry skin is thin and very sensitive to wind and weather, and its pores are small and thin; it cracks easily, as it is not flexible enough. Such skin needs to create maximum comfort and gentle treatment, it is better to use expensive varieties of soap. Normal skin is soft, smooth, has pores of medium size.

Hydrogen peroxide Hydrogen peroxide is the simplest representative of peroxides. Colorless liquid with a “metallic” taste, infinitely soluble in water, alcohol and ether. Ego is often used in everyday life as a bleach and antiseptic. When hydrogen peroxide is decomposed (when we treat a wound), water and gaseous oxygen are released. 2Н 2 О 2 \u003d О 2 + 2Н 2 О At not large doses, a small amount of oxygen is correspondingly released. In a small volume, pure oxygen is not dangerous, but in a large volume? And with a large amount, pure oxygen is toxic and can cause a pulmonary form of oxygen poisoning and a harmful effect on the central nervous system. The first exposure is accompanied by such symptoms: irritation of the lung tissue. It can begin with a slight irritation of the pharynx and subsequent coughing. In severe cases, prolonged burning in the chest and uncontrolled coughing may occur. Pulmonary oxygen poisoning can also cause a decrease in lung capacity and gas exchange capacity, although these complications are extremely rare. Symptoms of the second effect (toxic damage to the central nervous system) include: visual impairment (tunnel vision, inability to focus), hearing impairment (ringing in the ears, the appearance of extraneous sounds), nausea, convulsive contractions (especially facial muscles), hypersensitivity to external irritants and dizziness . But all this is possible only when using large volumes of hydrogen peroxide, and the usual 3% peroxide is incapable of this.

Extinguishing soda with vinegar The process of extinguishing soda with vinegar is used when mixing dough for buns and pancakes. Baking soda when exposed to high temperature or acidic environment gives an enhanced reaction for the release of carbon dioxide, which in turn leads to splendor and porosity. CH 3 COOH + NaHCO 3 \u003d CH 3 COONa + H 2 O + CO 2 The question "quench or not quench soda with vinegar during baking" is as eternal as the question: "what happened before - a chicken or an egg." However, delving into the literature, taking a break from a bunch of sites, including foreign ones, I came to the conclusion that a great many recipes of ancient Russian cuisine did not find a single one that mentioned soda because of the strength of years. Baking used to be in our country was mainly yeast, or without adding any lifting and loosening accelerators at all. So, baking soda was invented by the French chemist LeBlanc at the end of the 18th century. This invention came to Russia much later, after receiving a new method for its manufacture. As soon as the Russian housewives had a product like soda, they began to use and use it in cooking. Why was it decided to extinguish soda? Yes, simply because our tradition is everything “with heat, with heat” in this case - it is only harmful. Quick baking soda in hot baking has a very unpleasant “soapy” taste. What was “corrected” by its quenching, namely, by adding boiling water to the soda or fermented milk products. For pancakes, this method now gives very good results. However, you can imagine what will happen with the shortcrust pastry if you pour a glass of boiling water there? The answer is obvious. Therefore, it was thought up to replace boiling water or dairy products with diluted 9% vinegar or lemon juice.

Conclusion We can observe many chemical reactions not only in chemistry lessons, but also in everyday life. These reactions are not only safe (subject to safety rules), but some of them are useless. For example: extinguishing soda with vinegar, any skilled chef would say that this is a waste of time. But without reactions such as hydrolysis and combustion, we simply have no idea of \u200b\u200bfurther existence. During the course of these chemical reactions, gases are released. They are safe (in a certain amount). When using chemicals in everyday life, compliance with safety regulations is necessary.

Sources of information 1. Kritsman, VA, Stanzo, VV Encyclopedic Dictionary of the Young Chemist [Text] -M. : Pedagogy, Lavrova, S.A. Entertaining chemistry [Text] -M. : White City, Ryumin, V. Entertaining Chemistry [Text] -M.: Center Polygraph, Kurdyumov, G.M. Question on Chemistry [Text] -M. : World, Binom, Guzey, L.S., Kuznetsov, V.N. New reference book on chemistry [Text] -M. : Ursa Major, Wikipedia [Electronic resource] - Access mode: ru.wikipedia.org 7. Egorova, A.S. Chemistry tutor [Text] -M. : Phoenix, Chemistry and Life [Electronic resource] - Access mode: http: //www.hij.ru 9. Chemistry around us [Electronic resource] - Access mode:

