At constant temperature, the volume occupied by a gas is inversely proportional to its pressure.
Robert Boyle - shining example a gentleman scientist, a son of a long-gone era when science was the domain of exclusively wealthy people who devoted their leisure time to it. Most of Boyle's studies relate to modern classification to the category chemical experiments, although he probably considered himself natural philosopher(theoretical physicist) and naturalist(experimental physicist). Apparently, he became interested in the behavior of gases after seeing the design of one of the world's first air pumps. Having designed and built another, improved version of his double-sided air-vacuum pump, he decided to investigate how increased and decreased gas pressure in a sealed vessel to which his new apparatus was connected affected the properties of gases. Being a gifted experimenter, Boyle at the same time adhered to very new and unusual views for that era, believing that science should come from empirical observations, and not be based solely on speculative and philosophical constructs.
In Boyle’s formulation, the law literally sounded like this: “Under the influence of an external force, a gas is elastically compressed, and in its absence it expands, while linear compression or expansion is proportional to the elastic force of the gas.” Imagine that you are squeezing an inflated balloon. Since there is enough free space between the air molecules, you can easily, by applying some force and doing some work, compress the ball, reducing the volume of gas inside it. This is one of the main differences between gas and liquid. In a bead of liquid water, for example, the molecules are packed tightly together, as if the bead were filled with microscopic pellets. Therefore, unlike air, water does not lend itself to elastic compression. (If you don’t believe me, try pushing a tightly fitting cork inside the neck of a bottle filled with water up to the cork.) Boyle-Mariotte law, along with Charles’ law, formed the basis of the Equation of State of an Ideal Gas.
J. Trefil calls it “Boyle’s law”, but we preferred what was accepted in Russian tradition name of the law. — Note translator.
See also:
Robert Boyle, 1627-91
Anglo-Irish physicist and chemist. Born in Lismore Castle, Ireland, becoming the fourteenth child of the Earl of Cork, a famous adventurer of the era of Queen Elizabeth. After graduating from the privileged Eton School, where he was one of the first students among the “young gentlemen,” he went on a many-year journey through continental Europe, during which he continued his education at the University of Geneva. Returning to his homeland in 1648, he equipped a private laboratory and began physical and chemical research on its basis. In 1658 he moved to Oxford, where Robert Hooke became his student and laboratory assistant. cm. Hooke's Law), future scientific secretary of the Royal Society. By the way, Boyle was one of the founders and co-founders of the Royal Society, which grew out of a circle of young Oxford scientists. Carried out a number of innovative chemical experiments, including experiments to study in detail the properties of acids and bases. According to some reports, he was the first to put forward a hypothesis about the existence of chemical elements. Proved that air is necessary for combustion and breathing. In addition to his studies in science, he was a co-founder and shareholder of the East India Company and was actively involved in missionary work in the hope of converting the inhabitants of the eastern colonies of the British Empire to Christianity.
) Other sources: MESBE
Boyle-Marriott law , connecting changes in the volume of a gas at a constant temperature with changes in its elasticity. This law, discovered in 1660 by the physicist Boyle and later, but, independently of him, by Mariotte in France, in its simplicity and certainty it occupies a very important place in science, although later research has shown the existence of deviations from it and that the law actually applies to the so-called ideal gas. The history of its discovery is very instructive. Franciscus Linus, professor of mathematics at Lüttich (1595-1675), did not admit that air, such a mobile and light substance, could support a column of mercury in a barometric tube, although Galileo's student Evangelista Torricelli (1608-1647) undoubtedly proved that that it is atmospheric pressure that is the cause of this phenomenon. Until that time, everyone assumed that nature abhors a vacuum (horror vacui) and that therefore mercury, water and all sorts of liquids rush into empty tubes. When it turned out that the water in the tube followed the pump piston only to a height of slightly more than 30 feet, Galileo decided that the fear of emptiness had a limit. Lin explained that the mercury was held in the tube by invisible threads (funiculus) and that he himself felt these threads when he closed the upper hole of the tube with his finger, which was then filled with mercury and turned the lower end into a cup with mercury; in this case, the mercury in a sufficiently long tube dropped, but stopped at a certain height. This interpretation of Torricelli's experience by Lean prompted Boyle to make several new experiments, which he described in his “A defense of the doctrine touching spring and weight of the air” (London, 1662). To prove that air has the ability to resist, Boyle took a siphon-shaped tube sealed at the short end (Fig. 1). When mercury was poured into a long elbow, it compressed the air contained in the short elbow, the more significantly, the more mercury was poured into the other. When the mercury in the short elbow reached level AB, in the long one it was at level CD, which means that the elasticity of the compressed air was such that it could maintain a pressure of a mercury column with a height from AB to CD. And since this height in B.’s first experiments was equal to the height of mercury in the barometer, this proved that in the barometer the mercury column was supported by atmospheric air. Pouring various, larger and larger amounts of mercury into the long elbow of the tube, B. recorded the heights of the mercury column and the corresponding volumes of compressed air, but at first did not pay attention to their numerical ratios. His student Richard Townley, looking at the numbers in the table, noticed that the volumes of trapped air were inversely proportional to the pressures exerted on it. If the air first occupied 12 inches of length in the tube, and the mercury in both elbows was at the same height, then when so much mercury was poured into the long elbow that the air occupied only 6 inches of length, it turned out that the height of the supported column of mercury was 29 English. inches. At first, the air locked in a short elbow had the same elasticity as the atmosphere, which could maintain mercury in the barometer at 29 inches in height, and in the second case, the locked air was subject to atmospheric pressure and mercury pressure of 29 inches, that is, in total - pressure 29 x 2 inches: this means that when the volume of air became half as small, its elasticity became twice as great. After this, Boyle repeatedly repeated and diversified his experiments and proved that the same law applies to cases of increasing air volume.
To do this, he used a cylindrical vessel (Fig. 2), which was filled with mercury; immersing tube A with open ends until part AB remained above the mercury, equal to 1 inch in length, B. closed and sealed hole A and then raised the tube. At the same time, the volume AB increased and finally turned into the volume AD - twice as large; mercury rose to a height B "D, which was almost half less, 29¾ inches, the then height of mercury in the barometer. Obviously, the air contained in DA did not have sufficient elasticity to press on the surface D with such force as it had before pressed on B; the difference in elasticity in both positions of the tube has the measure of a column DB", whose length turned out to be 15⅜ inches. Therefore, the elasticity of air in double volume AD is 29¾ without 15⅜, i.e. 14⅜ or almost exactly half of the previous one. When the tube was raised so that the volume AD occupied a length of 10 inches, the height of the mercury DB" was found to be 26¾, therefore the elasticity of the air was measured by the difference 29¾-26¾, i.e. 3 inches, which is almost exactly 1/10 of the original elasticity A description of these experiments is in "New Experiments touching the spring of the air" (Oxf., 1660); "Continuation of Experiments" (Oxf., 1669), "On the rarefaction of air" (London, 1671); "Second continuation" (London, 1681), "General history of the air" (London, 1692). The French scientist Mariotte (Edme Mariotte, 1620-1684) carried out a series of experiments in exactly the same way and found the same law, which is usually called his name; only the English call it Boyle's law. See Mariotte's works: “Essay sur la nature de l'Air” (Paris, 1676), “Du mouvement des eaux et des autres fluides” (part. II, disc. 2). Whether Marriott knew about Boyle’s experiments cannot be answered positively, although it is known that Marriott was in relations with English scientists already in 1668. Be that as it may, Marriott performed the same experiments and measurements as Boyle, only with greater accuracy, and his experiments became better known. The tube (Fig. 1) received, as a device, the name Marriott, and the law was named after him, although for some time it has been rightly called the Boyle-Mariotte law; Perhaps it would be even fairer to add the name of Tonley. In any case, Marriott is so famous for his other works that, despite the evidence of the numbers, it is difficult to suspect him of the lack of independence of the work that led to the secondary discovery of an important physical law. The history of physics shows that very important laws discovered in one country could for a long time to be unknown to another; Thus, an important law concerning the strength of galvanic current, discovered by Ohm in Germany, was discovered again a few years later in France by the physicist Poulier.
As the volume of air decreases by two or three times, its density necessarily increases in the same ratio; the temperature of the gas when measuring its volume must be constant, otherwise cooling or heating it in itself can change the volume and elasticity; In addition, the air must not contain water or other liquids. Subject to all these conditions, the Boyle-Mariotte law should be expressed as follows: the volumes of a certain amount of dry air at a constant temperature are inversely proportional to the pressures exerted on it, and, consequently, to its elasticity, the density of air is directly proportional to this pressure; or, in short, the volume of air is inversely proportional to the pressure exerted on it. If we denote the initial volume of a gas by the letter v, and the pressure under which it is located by the letter p, if the compressed volume of the gas is v", and the pressure, always measured by the height of the mercury column, will be p"; then the law of B.-M. will be expressed by proportion: v: v" = p": p; whence pv = p"v", i.e. the product of the gas volume and the corresponding pressure is a constant value at a constant temperature. Other gases, as will be explained later, follow the same law. No matter how simple the experiments of Boyle and Mariotte seem, however, even with the low degree of accuracy of the instrumentation that was available at that time, they required the observance of many experimental precautions. Failure to observe proper rules was probably the reason for the various contradictory testimonies of later observers. For example, Bez observed in his experiments under the equator a decrease in the volume of air in a smaller ratio than an increase in its elasticity. Numerous experiments by Bugar at the same latitudes, on the contrary, confirmed the B.-M. law; in addition, the experiments of Amonton, Sgravesand, Fontana, Schuckburg led to the same conclusion.
But all the experiments of that time did not reach high pressures and were not so accurate that there was no doubt about the correctness of the law. Sulzer (“Mém. de Berlin,” vol. IX, 1753), and then Robison, concluded from their experiments that at pressures 7 or 8 times higher than atmospheric pressure, elasticity increases in a much smaller ratio than the volume decreases; but Winkler’s experiments (1765) again prove the applicability of the B.-M. law. up to 8 atmos. pressure. In this century (1826), Danish scientists Oerstedt and Svensen once again confirmed the validity of the law up to 8 atm. pressure; their other experiments, which extended to 70 atmospheres, were made using a less reliable method. But even within these close limits (up to 8 atm), some gases do not follow the B.-M. law. In the second half of the 18th century. Van Marum made sure that ammonia gas decreases in volume much faster than air; similar to that Oerstedt and Svendsen much later found sulfurous acid for the gas. In addition, it was discovered that both gases transform into a liquid state at slightly higher pressure; this property was later proven for other gases. Despretz, with even more precise experiments (“Ann. de Chim. et de phys.”, 2, XXXIV, 1827), became convinced that many gases do not follow the B.-M. law. even at pressures that are far from those at which liquefaction of gases occurs. Despres carried out experiments using a method similar to that first used by Van Marum. Two glass tubes, sealed at one end, one of which was filled with air and the other with another gas, were immersed with their open ends in a bath filled with mercury, placed at the bottom of a glass cylinder filled with water. Pressure was exerted on the water by means of a piston placed in the upper bottom of the cylinder; the water pressed on the mercury, which, entering the tubes, compressed the gases. Experiments made with such a device led Depres to the conclusion that ammonia, sulfur dioxide, hydrogen sulfide and sulfur dioxide gases at the same pressure occupy less volume than air. The accuracy of the measurements was so great that the difference between the compression of these gases and air was noticeable even when the volume of the latter was reduced by only half; Moreover, the volumes of these gases were less than half the initial volume. According to Depres' experiments, hydrogen gas is compressed equally with air to 1/15 of its original volume, but at twenty atmospheres of pressure the volume of hydrogen was greater than the corresponding volume of air. Dulong and Arago ("Mémoires de l'Académie des Sciences", vol. X, "Annales de Chim. et de Phys.", vol. XLIII, 1830) measured the compression of air to 27 atmospheres of pressure; their device consisted of a tube 1.7 m long, in which air was compressed, and connected to it by another, made up of 13 parts, each 2 meters long. This long, composite tube was attached to a wooden mast mounted inside a tall tower. Dulong and Arago found that the B.-M. law. true for air even when compressed to 1/24 of its original volume. Later, the French physicist Poulier carried out experiments using a method similar to that used by Oerstedt and Despres, but at high pressures, and concluded that oxygen, nitrogen, hydrogen, carbon monoxide and nitrogen oxide follow the same compression law up to 100 atmospheres as air, but that the six gases named below are compressed more than air and that the difference between their volumes and the volume of air increases with increasing pressure. These gases are: sulfurous acid, ammonia, carbon dioxide, nitrous oxide, oil and swamp gases.
In 1847 Regnault's extensive and accurate research on this subject was published (“Mémoires de l'Académie des sciences de Paris”, XXI, 1847), which, together with other physical work carried out on behalf of the French government, are described in the said memoirs under with the title “Relation des expériences entreprises par ordre de M. le ministre des travaux publics etc.” Taking advantage of the improvements in instruments and methods of observation introduced by his predecessors, Regnault added significant new improvements, eliminating the main difficulty in accurately measuring gradually decreasing volumes of gas. No matter how significant the length of the tube in which the gas was compressed in the experiments of Arago and Dulong was (1.7 meters), still at high pressures the volume of the gas became very small, and then any small inaccuracy in measuring the position of the mercury blocking the gas becomes increasingly and more perceptible relative to the constantly decreasing volume being measured. In his experiments, Regnault used a 3-meter-long tube to compress gases and, after measuring the full volume of gas and then compressing it to half the volume at a certain appropriate pressure, he again pumped gas into this tube until it was completely filled. The resulting large volume of gas, again under pressure, b O greater than the original, was brought back to half the volume by increasing the height of the mercury column in a long tube. Using this method, Regnault always measured large volumes at very high pressures (25 atmospheres for air); in addition, he took into account many other experimental precautions that ensured the accuracy of his conclusions. Regnault's experiments have proven that the important law of nature indicated by Boyle and Mariotte is not formulated mathematically precisely by the simple relationships they gave him, that the compression or decrease in the volume of air and nitrogen occurs in a slightly greater ratio than the increase in pressure on the gas or than the elasticity of the latter , and that for hydrogen the compression, on the contrary, is somewhat weaker than would be expected in the case of the exact applicability of the B.-M. law to it. Several figures taken from Regnault's memoirs, included in the following tablet, show that the observed retreats are generally small, but clearly increase with increasing pressure. The first two columns of the table show the height of the mercury column pressing on the gas, expressed in atmospheres (in Regnault in millimeters), and the height of 760 million mercury is taken as a measure of normal atmospheric pressure. The numbers in the third column show the quotients obtained by dividing the ratio of the initial volume of gas to the volume reduced by compression by the ratio of the latter pressure to the initial one. If we call the letters v, v 1 the initial and reduced volumes of gas, and the letters. p and p 1 are the corresponding pressures on the gas, then according to the B.-M. law. should be: v: v 1 = p 1: p, hence (v: v 1): (p 1: p) = 1, i.e., if both written relations are really equal, then the quotient of dividing one relation by another should be equal to 1. But the numbers in the third column are increasingly greater than 1 and slowly but constantly increasing:
Any number in the third column shows the quotient related to the reduction in air volume by half when the pressure moves from p (number of the first column) to p 1 (second column). From these numbers it is clear that the decrease in air volume occurs in a greater ratio than the increase in the corresponding pressure or elasticity of the gas. At first, both ratios differ little from each other, but when moving from 12 atm. by 24, the decrease in volume is 1.006366 times greater than the increase in pressure. A little calculation allows us to conclude that 10,000 cubic meters. sant. air at a pressure of 0.972 atm, being subjected to a pressure 24.9 times greater, will occupy a volume of 396 cubic meters. sant. instead of 401 k.s., as it should have been if the law of B.-M. accurately expressed the law of nature.
The compression of nitrogen represents similar, but somewhat smaller deviations from the B.-M. law, and since atmospheric air consists of oxygen and nitrogen, Regnault concluded that oxygen is compressed more than nitrogen and air. The following tablet contains the numbers obtained during experiments I with hydrogen; The column numbers have the same meaning as in Table A.
Since all the numbers in the third column are less than one and are constantly decreasing, the volume of compressed hydrogen is always greater than what would follow according to the B.-M. law, and with increasing pressure this deviation increases. According to Regnault, hydrogen compresses like a spring, less and less as pressure increases. As for carbon dioxide , relatively easily compressible, which, like air, represents a more rapid decrease in volume than an increase in elasticity, then it deviates from the law already at relatively weak pressures at ordinary temperature, but, being heated to the boiling point of water (100 ° C.), shows much smaller retreats. If from the extremely accurate experiments of Regnault it should be concluded that the law of B.-M. with very insignificant deviations is applied only to some gases at pressures far from the liquefaction point and at a significantly high temperature, then the study of the issue is not exhausted by these results. The experiments of Boyle and Regnault are separated by a period of time of almost 200 years. The properties of gases were studied in many respects during this period of time, the list of liquefiable gases was constantly increasing, and several years ago, through the works of Pictet and Cailletet, a final generalization was made that with a decrease in the volume of gases through pressure and with a decrease in their temperature, they all turn into liquid . At the same time, research on gas compression was supplemented by other scientists who compressed gas at pressures far exceeding the 25 and 30 atmospheres that Regnault and his immediate predecessors stopped at. It was mentioned above that Poulier had already brought pressures up to 100 atm, but his experiments were not arranged in such a way that they could find an answer to the meaning of the B.-M law. at high pressures. This answer is given by the experiments of Natterer, Kalete and Amag for strong pressures and the experiments of D.I. Mendeleev for weak ones. Amaga installed his device at the bottom of the shaft, which was about 400 meters (about 190 fathoms) deep. Measurements of the volume of gas at such a depth and the enormous height of the pressing mercury column were accompanied by such great technical difficulties that only the compressibility of nitrogen was directly studied. The law of compression of other gases in comparison with nitrogen was found by Amaga using the method of Depres and Poulier. In Amag's experiments, the pressure reached 430¾ atmospheres, and the volume of nitrogen decreased only 335¾ times. Calete lowered his device into an artesian well 500 meters deep (about 230 fathoms); the height of the pressing mercury column was gradually increased as the device was lowered. The tube in which the gas was compressed was gilded inside; mercury, entering it, amalgamated the gold, so that a trace remained on the gilding, a limit between the gas and mercury, by which it was possible to measure the volume occupied by the compressed gas. In addition, Calete conducted experiments on the compression of air and hydrogen in a special device, in which the pressure was brought to 605 atmospheres. These experiments were preceded by the research of Natterer (1851-1854), who, using a special pressure pump device, brought the pressure on the gas to 2790 atmospheres. The gas was concentrated in a thick-walled steel vessel, which was equipped with a well-made valve, gradually loaded as the elasticity of the gas increased, which was measured by the weight of the load on the valve. At the end of the compression of the gas, it was passed in parts into another vessel of a certain volume, where it assumed an elasticity equal to one atmosphere, and a successive decrease in the elasticity of the compressed gas was determined, at first rapid, then slowing down more and more. The numbers obtained from these measurements provided a means of determining the elasticity of gases corresponding to its compression. The combination of all these experiments, in comparison with those of Regnault, led to the conclusion that all gases, with the exception of hydrogen, undergo such changes in volume v and elasticity p, starting from one atmosphere, that the product vp decreases until the pressure or elasticity reaches a certain limit, and that with further increase in pressure this product vp increases. In the first period, gases are compressed more than should be according to the B.-M. law, in the second period - less. The limits, i.e., the number of atmospheres of pressure at which the compression value should be obtained according to the B.-M. law, are shown differently by different researchers, but there is no doubt that for each gas there is a special such limit; only hydrogen, at all tested pressures, is compressed less than it should according to the B.-M. law. It remained to supplement these studies by studying the relationship between the elasticity and volume of gases at pressures less than atmospheric, that is, in rarefied air; according to the low-precision experiments of Boyle and Mariotte, their law is also true for rarefied air. An accurate study of the law of compression of rarefied gases was made by D. I. Mendeleev with the collaboration of M. L. Kirpichev (experiments of the Imperial Russian Technical Society, “On the elasticity of gases” by D. Mendeleev, part 1, St. Petersburg, 1875, in 4°). This work and others related to it were carried out at the expense of the Technical Society; Using the same funds, the aforementioned essay was published, which describes the author’s techniques and instruments for measuring the elasticity and volumes of gases. Experiments were carried out on air, hydrogen and carbon dioxide. Below is one series of experiments, from which the relationships between the volumes of very rarefied air and its elasticity are visible.
From this it can be seen that with a decrease in pressure on a gas, its volume increases in a smaller ratio than the elasticity decreases, therefore, and vice versa: with an increase in pressure, the volume decreases in a smaller ratio. In fact: the second pressure is 7.71 times less than the first, and the second volume is only 7.38 times more than the first; the third pressure is 2.35 times less than the second, and the third volume is 1.92 times more than the second. This means that the compression and expansion of air at very low pressures deviates from the B.-M. law. in the same direction, as with very strong pressures; A similar thing happened for carbon dioxide. Amaga and Zillestrom worked on the same issue, Regno also made several measurements with air at an elasticity of 300 millimeters. Regnault and Zillestrom came to the conclusion that rarefied air deviates from the B.-M law. in the same direction as at pressures slightly above atmospheric; Amag’s experiments did not lead him to reliable results (see the critical assessment of the experiments of R. and Z. made by D. I. Mendeleev in the essay “On the Elasticity of Gases,” §§ 82, 92, 94.)
Summarizing everything that has been said regarding air, one can see that in a rarefied state it compresses less than it follows according to the B.-M. law, that at a density near atmospheric and higher, air compresses more than according to the B.-M. law, and, finally, at a very high density, it again retreats in the same direction as at a very low one. When moving from retreats in one direction to retreats in the other, the air must necessarily be compressed according to the B.-M. law, and this happens only twice, ranging from the lowest elasticity studied (about ⅓ mill.) to the highest (2700 atmospheres). Other gases probably follow the same law of variable compression, except hydrogen, which is constantly compressed less than according to the B.-M law.
Doubts have long been raised about whether gases could follow the B.-M. law. at very high pressures. Since during compression the density of the gas constantly increases to the same extent, it would be possible to reach the point that the compressed gas would be denser than the densest metal, i.e., that the gas brought by compression to a certain volume would be heavier, for example, platinum taken in the same volume. Unlimited compression of gas cannot be allowed for the reason that the substance of the gas, which itself occupies a certain part of the space, thereby sets a limit to compression. Modern chemistry (see Mendeleev, “On the Elasticity of Gases,” pp. 8-12) leads to considerations that do not allow the possibility that a gas can be brought to a very high density by compression. But in fact, it is a noted fact that all tested gases at high pressures occupy a volume not as small as would follow according to the B.-M. law, and that the deviations from this law are more significant, the greater the pressure; this fact shows that the reduction in volume is approaching a certain limit. For some gases at ordinary temperatures such a limit has been found, since these gases turn into liquid, and liquids at the highest pressures only decrease very slightly in volume. Other gases, which do not turn into liquid from one compression without a more or less significant decrease in temperature, deviate more and more from the B.-M law. Hydrogen at 3000 atm. pressure occupies a volume only 1000 times smaller than the original one, i.e. at this pressure its volume is three times more than would be expected if the B.-M law is accurate. Several experiments by Regnault on the compression of gases at the boiling point of water show that as the temperature rises, deviations from the B.-M. law. become less; this circumstance led him to the conclusion that an increase in temperature brings the gas closer to an ideal state in which it follows the B.-M. law, but this concept of an ideal gas is not yet sufficiently substantiated. In conclusion, it must be said that the B.-M. law, actually expressing the compression of gases only in certain limiting cases, nevertheless served as the starting point for the study of their properties. Together with Gay-Lussac's law, which relates to the expansion of gases from heat, it presents a mathematical formula that must be modified in order to fully represent the phenomenon of changes in the volume of gases. The Van der Wals formula (see this word) already penetrates deeper into the nature of gases.
Despite the many experimental work on the compression of gases, science can expect even new, even more extensive research. It would be desirable to see the precise and difficult studies of highly expanded gases made by D.I. Mendeleev, leading to important conclusions, repeated and disseminated. Regnault's experiments will remain guiding for a long time, but the accuracy of our time may seem insufficient in the near future.
Scientists studying thermodynamic systems have found that a change in one macroparameter of the system leads to a change in the rest. For example, an increase in pressure inside a rubber ball when it is heated causes an increase in its volume; An increase in the temperature of a solid leads to an increase in its size, etc.
These dependencies can be quite complex. Therefore, first we will consider the existing connections between macroparameters using the example of the simplest thermodynamic systems, for example, for rarefied gases. The experimentally established functional relationships between physical quantities for them are called gas laws.
Robert Boyle (1627-1691). A famous English physicist and chemist who studied the properties of air (mass and elasticity of air, the degree of its rarefaction). Experience has shown that the boiling point of water depends on pressure environment. I also studied elasticity solids, hydrostatics, light and electrical phenomena, first expressed his opinion about the complex spectrum white light. Introduced the concept of “chemical element”.
First gas law was discovered by the English scientist R. Boylem in 1662 while studying the elasticity of air. He took a long bent glass tube, sealed at one end, and began to pour mercury into it until a small closed volume of air formed in the short elbow (Fig. 1.5). Then he added mercury to the long elbow, studying the relationship between the volume of air in the sealed end of the tube and the pressure created by the mercury in the left elbow. The scientist’s assumption that there is a certain relationship between them was confirmed. Comparing the results obtained, Boyle formulated the following position:
There is an inverse relationship between the pressure and volume of a given mass of gas at a constant temperature:p ~ 1/V.
 |
| Edm Marriott |
Edm Marriott(1620—1684) . French physicist who studied the properties of liquids and gases, collisions of elastic bodies, pendulum oscillations, and natural optical phenomena. He established the relationship between the pressure and volume of gases at a constant temperature and explained on its basis various applications, in particular, how to find the altitude of an area using barometer readings. It has been proven that the volume of water increases when it freezes.
A little later, in 1676, the French scientist E. Marriott independently of R. Boyle, he generally formulated the gas law, which is now called Boyle-Mariotte law. According to him, if at a certain temperature given mass gas occupies volume V 1 at pressure p1, and in another state at the same temperature its pressure and volume are equal p2 And V 2, then the following relationship is true:
p 1 /p 2 =V 2 /V 1 or p 1V 1 = p2V 2.
Boyle-Mariotte Law : if at a constant temperature a thermodynamic process occurs, as a result of which the gas changes from one state (p 1 andV 1)to another (p2iV 2),then the product of pressure and the volume of a given mass of gas at a constant temperature is constant:
pV = const.Material from the site
A thermodynamic process that occurs at a constant temperature is called isothermal(from the gr. isos - equal, therme - warmth). Graphically on the coordinate plane pV it is represented by a hyperbole called isotherm(Fig. 1.6). Different temperatures different isotherms correspond - the higher the temperature, the higher on the coordinate plane pV there is a hyperbola (T 2 >T 1). It is obvious that on the coordinate plane pT And VT isotherms are depicted as straight lines, perpendicular to the temperature axis.
Boyle-Mariotte Law installs relationship between pressure and volume of gas for isothermal processes: at constant temperature, the volume V of a given mass of gas is inversely proportional to its pressure p.
In lesson 25 "" from the course " Chemistry for dummies"We will consider the law connecting the pressure and volume of a gas, as well as graphs of the dependence of pressure on volume and volume on pressure. Let me remind you that in the last lesson “” we looked at the structure and principle of operation of a mercury barometer, and also gave a definition of pressure and considered its units of measurement.
(1627-1691), to whom we owe the first practically correct definition chemical element(we learn in Chapter 6), was also interested in the phenomena occurring in vessels with rarefied air. Inventing vacuum pumps for pumping air out of closed containers, he drew attention to a property familiar to anyone who has ever inflated the bladder of a soccer ball or gently squeezed a balloon: the more the air in a closed container is compressed, the more it resists compression. Boyle called this property " springiness» air and measured it using a simple device shown in Fig. 3.2, a and b.

Boyle trapped some air in the closed end of a curved tube with mercury (Fig. 3-2, a) and then compressed this air by gradually adding mercury to the open end of the tube (Fig. 3-2, b). The pressure experienced by the air in the closed part of the tube is equal to the sum of atmospheric pressure and the pressure of a column of mercury of height h (h is the height to which the mercury level in open end tube exceeds the mercury level at the closed end). The pressure and volume measurements obtained by Boyle are given in table. 3-1. Although Boyle did not take special measures to maintain a constant temperature of the gas, apparently, in his experiments it changed only slightly. However, Boyle noticed that the heat from the candle flame caused significant changes in the properties of the air.
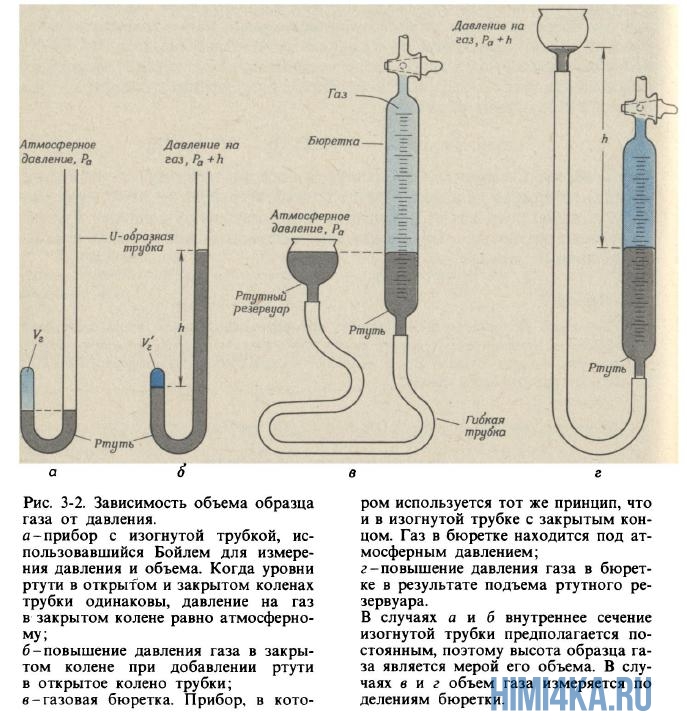
Analysis of data on the pressure and volume of air during its compression
Table 3-1, which contains Boyle's experimental data on the relationship between pressure and volume for atmospheric air, is located under the spoiler.


After the researcher receives data similar to those given in table. 3-1, he's trying to find Mathematical equation, connecting two mutually dependent quantities that he measured. One way to obtain such an equation is to plot graphically the dependence of various powers of one quantity on another in the hope of obtaining straight line graph. General equation straight line looks like:
- y = ax + b (3-2)
where x and y are interrelated variables, and a and b are constant numbers. If b is zero, a straight line passes through the origin.
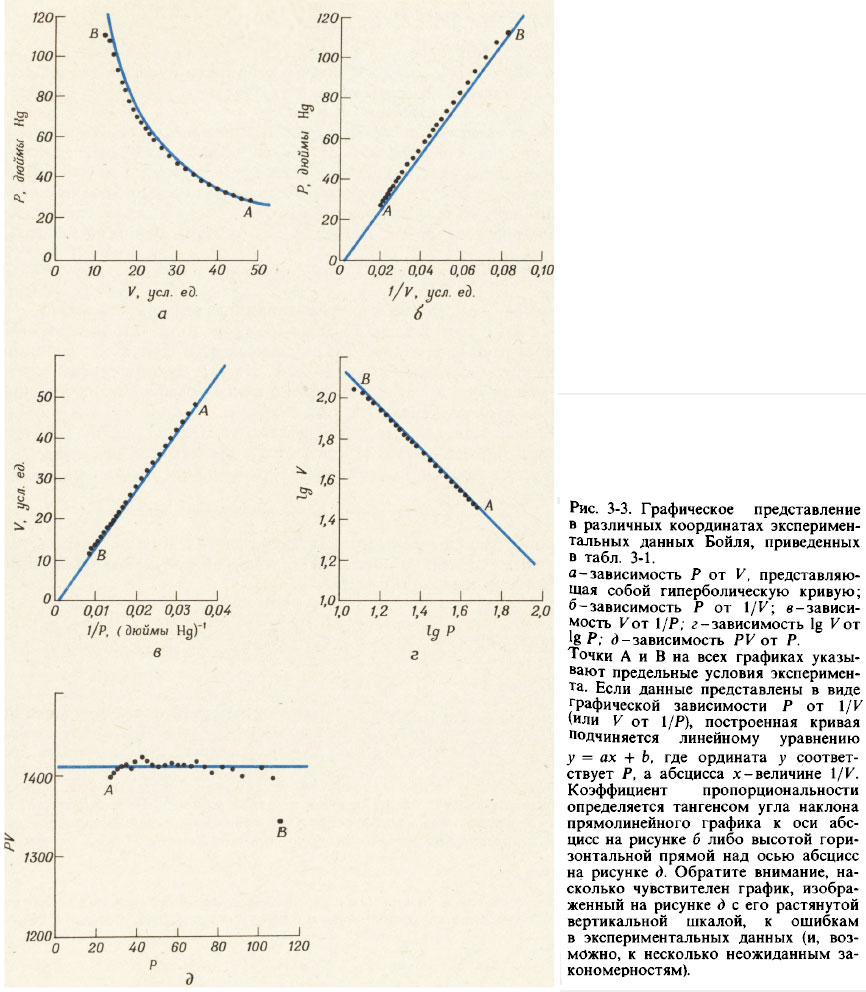
In Fig. 3-3 shows various ways of graphically presenting data for pressure P and volume V given in table. 3-1. Graphs of P versus 1/K and V versus 1/P are straight lines passing through the origin. The graph of logarithm P versus logarithm V is also a straight line with a negative slope, the tangent of which is equal to - 1. All three of these graphs lead to equivalent equations:
- P = a / V (3-3a)
- V = a / P (3-3b)
- log V = log a - log P (3-3v)
Each of these equations represents one of the options Boyle-Mariotte law, which is usually formulated like this: for a given number of moles of a gas, its pressure is proportional to its volume, provided that the temperature of the gas remains constant.
By the way, you probably wondered why the Boyle-Marriott law is called by a double name. This happened because this law, independently of Robert Boyle, who discovered it in 1662, was rediscovered by Edmus Marriott in 1676. Just like that.
When the relationship between two measured quantities is as simple as in this case, it can also be established numerically. If each value of pressure P is multiplied by the corresponding value of volume V, it is easy to verify that all products for a given gas sample at a constant temperature are approximately the same (see Table 3-1). Thus, we can write that
- P V = a ≈ 1410 (3-3g)
Equation (3-3g) describes the hyperbolic relationship between the values of P and V (see Fig. 3-3, a). To check that the graph of the dependence of P on V, constructed from experimental data, really corresponds to a hyperbola, we will construct an additional graph of the dependence of the product P V on P and make sure that it is a horizontal straight line (see Fig. 3-3,e) .
Boyle established that for a given amount of any gas at a constant temperature, the relationship between pressure P and volume V is quite
is satisfactorily described by the relation
- P V = const (at constant T and n) (3-4)
Formula from the Boyle-Mariotte law
To compare volumes and pressures of the same gas sample at different conditions(but constant temperature) it is convenient to imagine Boyle-Mariotte law in the following formula:
- P 1 ·V 1 = P 2 ·V 2 (3-5)
where indices 1 and 2 correspond to two different conditions.
Example 4. Plastic bags of food delivered to the Colorado Plateau (see example 3) often burst because the air in them expands when rising from sea level to an altitude of 2500 m, under conditions of low atmospheric pressure. If we assume that inside the bag at atmospheric pressure corresponding to sea level, contains 100 cm 3 of air, what volume should this air occupy at the same temperature on the Colorado Plateau? (Assume that wrinkled pouches that do not restrict air expansion are used to deliver products; the missing data should be taken from Example 3.)
Solution
Let's use Boyle's law in the form of equation (3-5), where index 1 will refer to conditions at sea level, and index 2 to conditions at an altitude of 2500 m above sea level. Then P 1 = 1.000 atm, V 1 = 100 cm 3, P 2 = 0.750 atm, and V 2 should be calculated. So,
- P 1 ·V 1 = P 2 ·V 2
- 1.000 atm 100 cm 3 = 0.750 atm V 2
- V 2 = 133 cm 3
I hope that after studying lesson 25 "" you will remember the dependence of the volume and pressure of a gas on each other. If you have any questions, write them in the comments. If there are no questions, then move on to the next lesson.
We begin the study of the relationship between the parameters characterizing the state of a given mass of gas by studying gas processes that occur while one of the parameters remains unchanged. English scientist Boyle(in 1669) and French scientist Marriott(in 1676) discovered a law that expresses the dependence of pressure changes on changes in gas volume at constant temperature. Let's carry out the following experiment.
By rotating the handle we will change the volume of gas (air) in cylinder A (Fig. 11, a). According to the pressure gauge reading, we note that the gas pressure also changes. We will change the volume of gas in the vessel (the volume is determined by scale B) and, noticing the pressure, we will write them down in the table. 1. It can be seen from it that the product of the volume of a gas and its pressure was almost constant: no matter how many times the volume of the gas decreased, the same number of times its pressure increased.
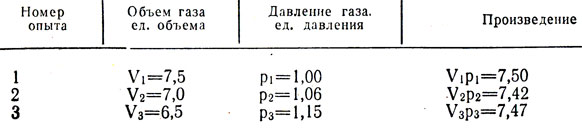
As a result of similar, more accurate experiments, it was discovered: for a given mass of gas at a constant temperature, the gas pressure changes in inverse proportion to the change in gas volume. This is the formulation of the Boyle-Mariotte law. Mathematically, for two states it will be written as follows:
![]()
The process of changing the state of a gas at a constant temperature is called isothermal. The formula of the Boyle-Mariotte law is the equation of the isothermal state of a gas. At constant temperature, the average speed of molecules does not change. A change in the volume of a gas causes a change in the number of impacts of molecules on the walls of the container. This is the reason for the change in gas pressure.
Let us depict this process graphically, for example for the case V = 12 l, p = 1 at.. We will plot the gas volume on the abscissa axis, and its pressure on the ordinate axis (Fig. 11, b). Let's find the points corresponding to each pair of values of V and p, and by connecting them together, we will obtain a graph of the isothermal process. The line depicting the relationship between the volume and pressure of a gas at constant temperature is called an isotherm. Isothermal processes do not occur in their pure form. But there are often cases when the gas temperature changes little, for example, when a compressor pumps air into cylinders, or when a combustible mixture is injected into the cylinder of an internal combustion engine. In such cases, calculations of gas volume and pressure are made according to the Boyle-Mariotte law *.
