A rather large proportion of the mass of dry mycelium of fungi is their cell wall, namely 5 to 15%. Its composition varies greatly and is often very specific for certain taxonomic groups of fungi. This can be seen from examples of its composition in representatives of yeast, chytrid and mucosal fungi - zygomycetes, given in Table. 1.3 (Aronson, 1965).
* (The amount of ash without phosphates.)
Structurally, the shells of fungi are built on the basis of a two-phase system, in which there are microfibrils included in the amorphous mass of the matrix. According to the data of electron microscopy, it consists of at least two layers with different fibril orientations. The inner layer is usually oriented along the main axis of the cell, the outer ones are at an angle to it (Fig. 1.1). In yeast, the shell is usually multilayered, with mannan localized in the outer thick layer, and glucan in the inner thin layer. In aquatic fungi, such as Allomyces, the shell forms false partitions - pseudosepta, resembling the spokes of a wheel. In marsupials and basidiomycetes, real partitions are observed - septa. In marsupials, septa between cells usually have one simple pore, on both sides of which, in the process of its formation, one can see a pair of osmophilic Voronin bodies. In basidiomycetes, these pores are often very complex, equipped with caps - parenthosomes (Fig. 1.2). Such pores have been found in the fruiting bodies and rhizomorphs of a number of higher basidiomycetes (Moore, 1965; Burnett, 1968). However, it is still not entirely clear whether this difference in the structure of the septal pores of asco- and basidiomycetes should be attributed to their taxonomic affiliation or to the haploid and homokaryotic structure of the marsupial genome and to the di- and heterokaryotic nature of basidiomycetes. There are no such studies yet, but their significance for evolutionary and taxonomic constructions in the kingdom of fungi is very fundamental.
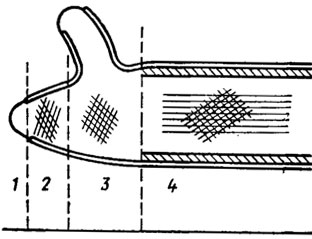

Many new things have been added to the study of fungal septal pores by studies at the electron microscopic level (Flegler et al., 1976; Kamaletdinova and Vasiliev, 1982). First, it became known that dolipores of basidiomycetes are structures apparently hermetically isolating mycelial cells from each other until they begin to form fruiting bodies (Flegler et al., 1976). This isolation is carried out by soluble proteolytic enzymes and at the same time by osmophilic (containing proteins and lipids) bilateral plugs, which disappear by the time the fruiting bodies are formed. Their disappearance is accompanied by perforations of the parentosomes and the communication between the hyphae cells is restored.
Recent observations on septa in the fruiting bodies of discomycetes (eg, Peziza badia - Kamaletdinova, Vasiliev, 1982) have shown that similar cell-dissociating hyphal structures also exist in the class of marsupial fungi. Voronin bodies take part in their formation, which are formed in flask-shaped invaginations of the cell membrane, the osmophilic content of which (Voronin body) is released near the septum and is located at the septum opening, gradually penetrating into it, while creating a closing device. Further penetration of the Voronin body into the overlying hymenial layer is apparently prevented by a special perforated structure lying above the pore of the septum in the mother cell of the future bursa, which is completely isolated from the penetration of submicroscopic organelles from the subhymenial layer. Similar isolating structures are also observed in the paraphyses formed in the fruiting bodies.
Similar structures are also found in the sporulating mycelium of imperfect fungi containing Voronin bodies, for example, in Arthrobotrys conoides. In the penicillin producer, the deuteromycete Penicillium chrysogenum, a structure was found in the septa that exactly corresponded to that found by Kamaletdinova and Vasiliev in the discomycete Peziza badia (Kurilowich et al., 1980).
The skeletal bases of fungal shells are composed of crystalline polysaccharides: cellulose, chitin, chitosan, mannan, glucans, and others. All of them have a linear structure with β-1,4 bonds of the initial components - monomers of hexoses, amino- and acetaminohexoses. According to the results of microchemical testing (ruthenium red staining), it was previously believed that pectin was present in the cell membranes of fungi. However, the results of chemical analysis did not confirm the galacturonic acid monomer included in the structure of pectin (Aronson, 1965).
Chitin and chitosan for most fungi are very characteristic in the composition of their shells as nitrogen-containing polymers. At the same time, fungal chitin is very similar to insect and crustacean chitin, which was confirmed by their X-ray diffraction pattern. However, there is less nitrogen in fungal chitin than in animals, and methylpentose, called mycetose, was found among its constituents. Chitin in fungi can be detected microchemically by the Van Wisseling method, using partial alkaline deacetylation and subsequent reaction with chitosan and X-ray diffraction. It was not found only in oomycetes, such as saprolegnia and peronospore fungi. Previously, it was believed that chitin is absent in yeast, but it is contained in cell partitions - septa of Saccharomycetes (Kulaev, 1975).
It has now been established that chitin can be found in Chitrydiales, Monoblepharidales, Protomycetales, Hyphochyiridiales, in all Endomycetales, Blastocladiales, Mucorales, Entomophthorales, in all marsupials and basidial fungi and Fungi imperfecti (deuteromycetes) derived from them. The exception is Oomycetes, in which cellulose is present in the shell of polysaccharides, which is completely absent in representatives of yeast fungi.
Recently, in connection with the discovery of the possibility of the practical use of fungal chitin for the synthesis of polymers, quite a few studies have appeared with data on a more subtle study of it in comparison with crustacean chitin (Table 1.4). Acetate and D-glucosamine are the least in Aspergillus niger, which is explained by the higher content of pentoses and glucose in the composition of β-glucan and two α-glucans of the fungus shell. An analysis of the diffraction patterns showed an identical crystal structure of chitin in fungi and crabs, with somewhat greater order in the latter. In addition, fungal chitin, in contrast to the lamellar structure of crabs chitin, had fibrous structure(Feofilova et al., 1980).
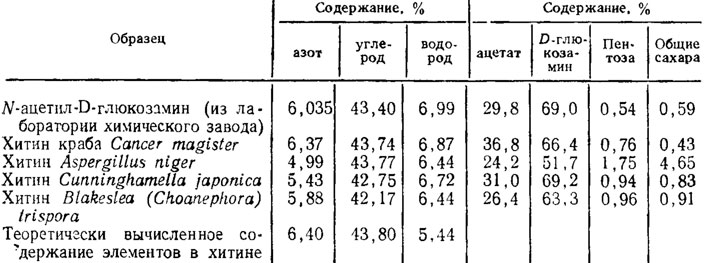
Chitosan, which replaces chitin in some mucosal fungi, is found during weak acid hydrolysis by the reaction for chitosan sulfate; in addition to Mucor rouxii, it was also found in Phycomyces blakesleeanus. The number of acetyl groups in it turned out to be different and varied in M. rouxii up to zero. Among other aminopolysaccharides, a galactosamine polymer with a free amino group capable of binding phosphates with a chitosan-type structure has been isolated from the cell walls of Neurospora crassa, N. sitophitla, A. niger, and Botrytls drierea (Aronson, 1965). A number of fungi contain polymers of amino sugars associated with mannan, glucan, and proteins.
Cellulose in fungal cell walls usually does not occur simultaneously with chitin. The exception is one Rhizidiomyces from the order of hypochytridia fungi, in which they were found simultaneously. Cellulose was found in oomycete fungi of the orders Acrasiales, Lagenidiales, Saprolegniales, Leptomitales, and Peronosporales (Aronson, 1965).
Among fungi living in the aquatic environment, cellulose is usually found only in groups with biflagellate zoospores. Blastocladiales and Monoblepharidales, which have uniflagellate zoospores, do not have it. The exception, which is Rhizidiomyces from the order Hyphochytridiales, which has both chitin and cellulose and is considered by Nable to be a transitional form between chitin-bearing Chytridiales and Blastocladiales and oomycetes containing cellulose, is understandable. This uniflagellated form has a pinnate zoospore flagellum rather than the whip-like flagellum of Blastocladiales and Monoblepharidales. It is curious that the structure of the villi of the pinnate flagella of oomycetes resembles the structure of the flagella of bacteria, while the whip-like flagella are completely similar to those of the flagellates.
Cellulose in fungi is easily detected microchemically with a Spitzer reagent or a reagent consisting of a solution of iodine in potassium iodide with the addition of a 70% sulfuric acid solution.
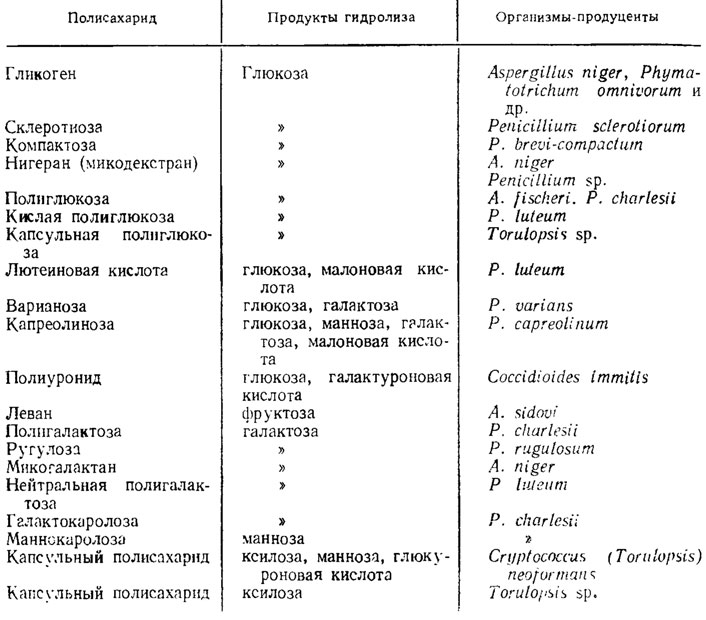
Glucans in mushrooms are very abundant and differ from cellulose in their structure. Their monomers are also glucose. However, the most studied yeast glucans have less crystalline structures than cellulose. The crystallinity of glucans increases due to the formation of hydroglucans during their treatment with sulfuric acid. Similar glucans associated with proteins have been found in yeast and Penicilliunt notatum (Aronson, 1965). Another glucan, which is part of the shells of fungi, is callose, similar to that found in the sieve tubes of higher plants and strongly stained with basic dyes, i.e., having an acidic nature, in contrast to cellulose, it has β-1,3-glucosidic bonds. A similar glucan staining with basic dyes has been found in Sclerofinia. Glucans are also found in Aspergillus fischeri, Allomyces macrogynus, Neurospora crassa. In fungi, there are also heteropolysaccharides composed of monomers of various sugars, especially common in the genus Penicillium. In yeast-like forms pathogenic to animals, like Coccidioides and Cryptococcus, similar but acidic polysaccharides are included in the capsules surrounding their cells. Examples of glucans and other polymerization products of monosaccharides and sugar acids, which are part of the cell membranes and storage substances of fungi, are given in Table. 1.5.
Mannans are polysaccharides composed of mannose monomers. they are found especially abundantly in yeasts, and they are frequent in yeast species that live on the surface of the cambial standing under the bark of trees. These forms include Hansenula, which lives under the bark of coniferous trees; in surrounding cell of these yeast capsules, the polysaccharide is present in the form of phosphomannan. This hydrophilic and slimy polysaccharide adheres together with yeast to the bristles covering the body of bark beetles, and in this way the yeast is transferred with their help from one tree to another (Wickerham and Barton, 1961). So far, mannans have not been found in hyphae-forming fungi, but mannose is found in the hydrolysates of their cell walls.
In fungi, there are also polysaccharides containing galactose, 6-deoxypentose, methylpentoses, most often fructose, especially in Mucoraceae. Pentoses of 6-deoxypentose, rhamnose and xylose, which was also found in the tinder fungus Polysiictus sanguineus, were found in cell membranes of Penicillium chrysogenum.
Polysaccharide-protein complexes have been found in yeasts, such as Candida albicans, the causative agent of thrush in infants. The mannan-protein complex has been found in Saccharomyces.
Lipids in fungi vary greatly depending on the conditions. environment and culture age. Sometimes their number reaches 35-36% of the mass of dry matter of cells. In yeast, more than 3% of lipids are found in their shells. They were also found in the shells of mucor fungi, for example, in Myco rouxii and Phycomyces, in the sporangiophores of which about 25% of lipids of their dry weight were found. They are probably contained there in formations of the cuticle type (Aronson, 1965).
Pigments are also included in appreciable amounts in the composition of the cell membranes of fungi. Pigments, especially the black pigment, melanin, often localized as a special layer, are very often found in the cell walls of the mycelium or in the shells of the spores of many fungi. Such a melanin layer is found in the ascospore membranes of Neurospora tetrasperma (Aronson, 1965).
Melanin is absent in fungi with low activity of polyphenol oxidase, which is involved in its biosynthesis, and with a predominance of active dehydrogenases in metabolism. Such fungi, which include representatives of the genera Fusarium, Trichothecium, Arthrobotris, Cephalosporium and many others, are most often characterized by a pink or orange color of sporulating structures, depending on the abundance of carotenoids, which take on the role of a light shield and an antioxidant, which belongs to melanin in dark-colored fungi. The relationship between the presence of carotenoids and high level The activity of dehydrogenases is explained by the fact that the optimal conditions of the redox regime for the action of dehydrogenases coincide with the conditions optimal for the biosynthesis of carotenoids and other products of the terpenoid shunt. Carotenoid pigments are no longer usually part of the cell membranes of fungi, but are localized either in cell membrane, or in lipid droplets dispersed in the cytoplasm. IN individual cases in fungi, there are very special pigments, such as ommochromes, eye pigments of insects, in the tinder fungus Pycnoporus (Polyporus) cinnabarinus (Shivrina, 1965) or phycobilins in russula species Russula emetica and R. paludosa (Efimenko, 1972), which are part of the photosynthesis system in blue- green and red algae.
Cell wall. It is a multilayer shell of 9...10 layers of different electron densities. A system of microfibrils embedded in an amorphous matrix forms the cell skeleton. Fibrils, depending on the species, may consist of cellulose, glucone, and chitin. Other polysaccharides, proteins, pigments, lipids serve as cementing agents that form chemical bonds with the microfibrillar part of the cell wall. The presence of such complexes provides selective permeability for some substances and blockade of others.
The supporting microfibrils of the cell wall and its matrix differ in the mechanism of formation and biosynthesis. The formation of fibrils and matrix occurs asynchronously; first of all, the fibrillar skeleton of the wall is regenerated. The biosynthesis of these two parts of the cell wall is carried out with the participation of enzymes.
The process of cell wall formation occurs in two ways: new material can either penetrate into the wall in a polarized manner or be evenly superimposed over its entire surface. In the first case, the formation of cylindrical cells occurs, in the second - spherical.
The cell wall serves as a protective device and protects the fungal cell from the effects of various environmental factors, for example, an osmotic barrier that determines selective permeability for various substances. It gives shape to the vegetative cells of hyphae and reproductive organs. On the surface of the cell wall and cytoplasmic membrane, enzymes are localized that convert polymers that are not absorbed by the cell (insoluble in water).
As a result of lysis, the cell wall of fungi can be destroyed under the influence of enzymes secreted by other cells and formed in the cell of the fungus itself.
The main components of the fungal cell wall are chitin, glucans, protein and fats. Nitrogenous and nitrogen-free polysaccharides form soluble and insoluble complexes with fatty substances. The basis of the cell wall is 4...6 monosaccharides, the ratio of which in different fungi varies slightly. The composition of polysaccharide fractions includes glucosamine, mannose, glucose, xylose, etc. It should be emphasized that the composition of the cell membrane various cells of the same fungus is not the same.
Protoplast- cell contents enclosed in the cell wall: Has a cytoplasmic membrane, endoplasmic reticulum, one or more nuclei with nucleoli, as well as mitochondria, ribosomes with RNA, lysosomes, the Golgi apparatus, vacuoles, a lamellar complex, secretory granules, and other structures and various inclusions.
cytoplasmic membrane. A thin three-layer membrane is located directly under the cell wall and separates it from the cytoplasm. The cytoplasmic membrane is selectively permeable to substances entering and leaving the cell. The cytoplasmic membrane contains up to 40% lipids and up to 38% proteins. Various forms of invagination and infringement of the cytoplasmic membrane are called mesosomes.
The main functional purpose of the cytoplasmic membrane is as follows: the implementation of the entry into the cell of various substances, enzymatic processing and release of metabolic products. The substances processed in the cytoplasmic membrane enter the protoplast of the cell and participate in the metabolism.
Endoplasmic reticulum. It consists of vesicles, tubules and vacuoles that serve as a kind of nutrient depot.
Mitochondria. Numerous mobile closed formations of an elliptical shape, with partitions, covered with a one- or two-layer shell. It is assumed that mitochondria, due to their own DNA circular structure, are capable of reproduction. Mitochondria are surrounded by a membrane on which enzymes are localized: pyruvate oxidase, succindehydrogenase, alkaline and acid phosphatase, peroxidase, etc. Mitochondria serve as energy generators in the cell. Depending on the conditions of cultivation and the physiological state of the cell, the shape of mitochondria and their number in the cell vary.
Ribosomes. Round grains of ribonucleoprotein nature, up to 200 cm in size, take part in the synthesis of cellular proteins. The number of ribosomes differs significantly in different types of fungi and depends on external factors, the age of the culture, etc.
Golgi apparatus. It is represented by a group of bubbles of very small diameter (0.000 002 ... O, OOO 01 microns) or parallel disc-shaped plates. This organoid is located in the cell at the site free from ribosomes.
Lysosomes. Derivatives of the Golgi apparatus are located between the cell membrane and the cytoplasmic membrane. They are granular formations surrounded by a single-layer lipoprotein membrane. They contain an enzyme that hydrolyzes protein and perform the function of protecting cells from the adverse effects of toxic substances of exogenous and endogenous origin.
Liposomes. Droplets of fatty substances surrounded by single-layer membranes.
Core. Located in the center or at the poles of the cell. Fungal cells can have single or multiple nuclei. They are responsible for hereditary functions. The shape of the nuclei is round or elongated. Each nucleus is surrounded by a two-layer porous nucleomembrane with a nucleolus of dense grains and thin fibrils. The nucleoli contain DNA in the chromosomes. Through anastomoses, nuclei can migrate from one cell to another.
Inclusions. There are numerous inclusions in fungal cells: volutin, glycogen, lipids, pigments, myeloid formations, salts of organic acids, amino acids, etc. It is believed that glycogen is responsible for endogenous respiration, and volutin serves as a reserve nutrient involved in energy processes.
It should be noted that in the process of vital activity, various metabolic products accumulate in fungal cells - antibiotics, enzymes, toxins, vitamins, etc.
All numerous morphological elements of microscopic fungi are divided into two groups: mycelium and spores. They come in various shapes and sizes. The morphological difference between spores and mycelium serves as an important differential feature in determining the type of fungus.
Mycelium. It is a narrow round tube, the diameter of which varies in micromycetes from one to several microns.
With abundant branching, mycelial hyphae, in contact with each other, can form fusions between cells - anastomoses. In the presence of a large number of them, the mycelium acquires a characteristic mesh appearance. The development of anastomoses is observed in various fungi with multicellular mycelium. Thanks to them, it is possible to move the cell nucleus from one cell to another and the transition from haploid to diploid mycelium. However, in most cases they carry out vegetative functions and develop in many forms with a lack of nutrition. The length of mycelial cells ranges from a few microns to tens and less often hundreds of microns.
The mycelium is surrounded by a double-circuit shell, which is more tender in young cultures. In the partitions dividing the mycelium into individual cells, there are pores through which the cytoplasm overflows during growth, and with it nutrients. There are many different inclusions in the cells: in the old, the cytoplasm becomes granular due to the many vacuoles. The young mycelium consists of elongated rectangular cells, the old - of short rounded or multifaceted. The mycelium, which has partitions, is called septate. However, in some lower fungi, the mycelium consists of hyphae devoid of transverse partitions, and is, as it were, one, highly branched giant cell with numerous nuclei and is called non-septate mycelium.
How does mycelium develop? A germ tube protrudes from the spore, which elongates and then is separated by a septum from the middle part, which includes the spore. The growth tubules then further elongate and receive a new septum, dividing into a distal, or apical, cell and a proximal, or internal one. Subsequently, the apical cell elongates and divides again, separating the second, younger than the first, internal cell. And so it goes on and on. In this process, the inner cells are only elongated, their transverse division rarely occurs, but side branches develop from them. At the distal end of the inner cell, a lateral protrusion is formed, which takes a cylindrical shape and is then separated by a septum from the cell that produces it. The new cell then grows into a lateral branch, growing and branching in the same way as the main one. Due to the development of branches throughout the main hypha, they are the older and more strongly developed, the closer to the base lies their discharge - acropetal branching.
The development of the non-septate mycelium proceeds in generally the same way, but without the formation of transverse septa. Growth occurs at the tips of the hyphae, where abundant protoplasm accumulates, filling the entire lumen, and in the more posterior parts, a significant development of the central vacuoles occurs. In a homogeneous environment, for example on the surface of nutrient gelatin, mycelial hyphae (both non-cellular and multicellular) grow evenly and radially, so that the mycelium has the shape of a circle growing from the edges. The central part in it is the oldest, even sometimes dead, and the peripheral part is the youngest.
With the general uniformity of the development of mycelium, which can be called typical, in some cases a number of specific features are observed as a macroscopic species and general growth and microscopic structure. The macroscopic appearance of the mycelium is determined primarily by aerial hyphae. In some cases, they form on the very surface of the substrate and partly inside it, and then the mycelium looks like a flat circle pressed against the substrate; in other cases, in addition, more or less abundant hyphae develop, rising into the air and giving the mycelium some resemblance, for example, to a piece of cotton wool rising above the substrate. The nature of growth may be different for the same fungus, depending on humidity, nutrition, etc. However, a number of forms of fungi have specific features, for example, the formation of a lush aerial mycelium - a wood destroyer.
The color of the mycelium is most often snow-white, but with age it acquires a brown color of different shades. This is due to the deposition of pigment in the cell walls and, less commonly, within the cell itself.
There are true mycelium and pseudomycelium. The latter is characterized by the fact that individual cells are not connected to each other and do not have a common membrane. Instead of true branching, a tree-like arrangement of cells is observed here.
In order to attach to the substrate and extract nutrients from it, in the course of evolution, some fungi formed organs specially designed for this: rhizoids and appressoria, which are taken into account when identifying fungi. Rhizoids are radicular, and appressoria are short, extended, sometimes lobe-shaped outgrowths of the mycelium.
Sclerotia, strands, rhizomorphs and chlamydospores are also modifications of mycelial growth.
Sclerotia are septate hyphae of fungi that form special bodies. During the formation of sclerotia, the membranes of the hyphae thicken and acquire a dark color. The wall of the hyphae of the outer layer of the sclerotium is strongly thickened, while inside the hyphae are thinner-walled and usually not colored. Sclerotia are protective adaptive bodies that allow the fungus to survive in the environment for a long time and ensure its resistance to various external factors: temperature, sunlight, etc. Mature sclerotia contain less moisture compared to mycelium and a lot of reserve substances - lipids, glycogen .
The sizes of sclerotia range from a few millimeters to several tens of centimeters, and the shape is the most diverse: spherical, irregular, in the form of straight or curved horns, etc.
The structure of sclerotia cells and the mechanism of their formation are different, but their formation occurs by increasing the branching of the mycelium and septation of hyphae. There are two ways of forming sclerotia: terminal - at the ends of hyphae; intercalary - in separate fragments of the main hyphae.
In many fungi, during the development of fruiting bodies and some vegetative structures, a false tissue is formed - plektenchyma (pseudoparenchyma). Unlike true parenchyma tissue, which results from cell division in three directions, plectenchyma is formed by plexus and accretion. If it consists of more or less isodiametric cells, then it is called paraplektenchyma; if it has a noticeable hyphae-like structure (elongated cells), then it is called prosoplectenchyma.
Mycelial strands- vegetative structure of linearly aggregated hyphae. The diameter of the mycelial strands depends on the number of hyphae that are concentrated around the central base.
In the simplest case, a small number of parallel hyphae are glued to each other with mucoid outer shells or enter into a stronger connection by fores. the formation of numerous short anastomoses. In other services teas, when the strands are massive, their hyphae receive a certain differentiation. The outer elements are thinner, forming, as it were, a bark around the central thick trunk.
rhizomorphs- more complex hyphae in terms of aggregation, which differ in different fungi in the intensity of growth of the central hyphae, the length of lateral branches, and the degree of differentiation of hyphae cells.
The outer parts of the rhizomorph are usually dark-colored and have a certain resemblance to the roots of higher plants. They are widespread in mushrooms with large fruiting bodies: in basidiomycetes, marsupials, etc.
The main purpose of mycelial strands and rhizomorphs is to ensure the spread of fungi in the substrate and the movement of nutrients along the hyphae.
Chlamydospores- these are changes in the mycelium in mature and old cultures at the ends or along its course. The main function of chlamydospores is not reproduction, but the preservation of the species. Their shape is usually round, oval or slightly elongated, the diameter exceeds the diameter of the mycelium. Some mushrooms have a double-walled wall, the surface is smooth or rough. Chlamydospores can occur at the ends of the mycelium, then they are called terminal, along the mycelium - interpolar (intermediate).
In old cultures, large clusters of chlamydospores are often observed in a bizarre shape resembling a rosary or a necklace. Young and mature chlamydospores are able to germinate. Old cells degenerate.
controversy. With the help of spores, fungi not only reproduce, but also spread in the environment. This is facilitated by the high resistance of spore shells to aggressive factors. Spores are divided into endospores, which are formed inside special receptacles - sporangia (bags), and exospores, located on the mycelium.
In perfect fungi, spores are divided into oospores, zygospores, ascospores, basidiospores, endospores, phialospores, chlamydospores. Spores of imperfect fungi are also divided into several groups according to size and origin. Endospores formed inside the mycelium by segmentation of the latter include thallospores, including arthrospores, chlamydospores and blastospores. In addition, imperfect fungi are characterized by the formation of conidia, macroconidia, aleuria (microconidia) and hemispores, which are considered imperfect conidia.
Hemispores are more firmly associated with the mycelium and represent one or two segments that lace off after the transverse division of the mycelial filament. Their shape is cylindrical, sometimes rounded or multifaceted, the shell is two-circuit.
MICROBIOLOGY, 2010, volume 79, no. 6, p. 723-733
UDC 582.281(047)
FUNGI CELL WALL: MODERN CONCEPTS ON THE COMPOSITION AND BIOLOGICAL FUNCTION
E. P. Feofilova1
institution Russian Academy Sciences Institute of Microbiology. S.N. Vinogradsky RAS, Moscow
Received November 5, 2009
The review is devoted to the little-studied surface structure of the cell of filamentous fungi - the cell wall (CS). Data are given on the methods of isolation and purity testing for the absence of cytoplasmic content in the CM fraction and on its chemical composition. Structural (framework) and intrastructural components of CS - aminopolysaccharides, a- and b-glucans, proteins, lipids, uronic acids, hydrophobins, sporopollenin and melanins are considered in detail. Special attention focuses on chitin, its new function in the anti-stress protection of cells, as well as the differences between this aminopolysaccharide of fungi and chitin of algae and Arthropoda. The phenomenon of hyphal apical growth and the participation of special microvesicles in fungal cell morphogenesis are discussed. Data on the enzymes involved in the synthesis and lysis of CS are given. In conclusion, the functional significance of the CS in fungi is discussed in comparison with the surface structures of higher eukaryotes.
Keywords: filamentous fungi, cell wall, isolation methods, chemical composition, apical growth, physiological functions, morphogenesis.
One of the key problems of modern biology is the question of how morphologically identical organisms are formed in the process of development, what biochemical mechanisms and what cellular structures are involved in this process, which has been taking place on our planet for billions of years. No less interesting are the data on how stress affects morphogenesis and which biopolymers control the external shape of cells. Research shows recent years, the growing hypha of filamentous fungi is a unique model that allows us to understand the process of cell formation and how the constancy of cell morphology is preserved in ontogenesis. A very large contribution to the study of this phenomenon was made by data on the study of the composition and biological function of the CL of filamentous fungi.
The plant CS was discovered in 1665, and the mushroom CS only at the beginning of the 18th century. However, this surface cellular structure was practically not studied for a long time, since it was believed that the CS performs only a "frame" function, and it was compared with the walls of a house that carry a supporting load. But at the beginning of the 20th century, the attitude towards CS changed dramatically, it began to be intensively studied, but mainly in plants and bacteria. However, the intensive development of mushroom growing and biotechnological industries, in which filamentous fungi were BAS producers, intensified the development
1 Address for correspondence (e-mail: [email protected]).
tie of scientific research on the study of the CS of fungi. Data on this surface structure for the period up to the 80s of the last century were summarized in the world's first book on fungal CS. In subsequent years, the bulk of the work fell on the end of the XX and beginning of XXI centuries. Basic research has been carried out on the taxonomy, chemical composition of CS, hyphal apical growth, chitin metabolism, antifungal drugs, hydrophobins, covalently bound proteins, enzymes involved in CS formation, hyphae branching, and CS lysis. These data were not summarized, but it is the discussion of them together that makes it possible to show that this cell surface structure carries a polyfunctional load and performs such important functions as, for example, protecting the cell from adverse factors, controlling morphogenesis, participating in reproduction processes, and determining antigenic and adhesive properties, control of the processes of dimorphism and the formation of resting fungal cells, the perception of an external signal and its transmission to the membrane and intracellular messengers. Given the above, we considered it appropriate to discuss the following data: the chemical composition of CS, the biological function of its main biopolymers, the structure of CS and intercellular interactions in fungal hyphae, the phenomenon of apical growth of hyphae, and the main enzymes involved in the formation and lysis of CS.
CHEMICAL COMPOSITION OF CELLULAR
WALLS OF FUNGI AND THE BIOLOGICAL FUNCTION OF ITS MAIN COMPONENTS
The study of the chemical composition of fungal CS begins with obtaining a pure fraction of this structure, i.e. purification of the COP from cytoplasmic contamination. The initial procedure - the destruction of fungal cells and the washing of cellular contents - is carried out at a low temperature (about 4-5 ° C) in order to prevent the destructive activity of degrading enzymes. Before cell destruction, they are frozen at a temperature liquid nitrogen. The destruction of cells is carried out either in special homogenizers, or on presses using the "hard pressure" method. Good results are obtained by further use of an ultrasonic disintegrator. Cellular contents are washed with cold water and repeated 4-5 times. For a more thorough removal of cytoplasmic contents, washing is used using NaCl, 8 M urea, 1 M ammonium, or 0.5 M acetic acid. At present, methods have been developed for isolating fungal CMs during their ontogenesis, and these methods differ significantly for mycelium and resting cells. Depending on the direction of research, in particular, when determining the polysaccharide composition, lipids are extracted from the CS using organic solvents, for example, chloroform and methanol in a ratio of 2: 1, sometimes additional treatment of the CS with sulfuric ether gives good results. This solvent dries the SC well and can sometimes replace freeze drying. The next step is to determine the purity of the resulting CS fraction. For this purpose, light and electron microscopy and specialized staining methods are used. The most commonly used reaction is based on the interaction of I3- with chitosan. Isolated pure CLs with Lugol's solution stain pink or purple, while intact CLs are red. Staining for the presence of nuclei with DAPI (4,6-ekt1yoto-2-rkepuCp-yo1) is also used. It should be emphasized that the isolation of the pure fraction of the CM is a very important procedure, on which the subsequent results of the analysis of its chemical composition depend. In this regard, the work is interesting, in which a glucan, more precisely, a chitosan-glucan complex, was found in the mycelium of a representative of Mucorales fungi, although all previous studies indicated the absence of glucan in the mycelium of Mucorales.
At present, the fungal CS components are divided into structural components (chitin, p-(1-3)-p(1-6)-glucans, p-(1-4)-glucan (cellulose), and intrastructural (they are called matrix) , which began to include mannoproteins, galacto-mannoproteins, xylo-mannoproteins, glucurono-
mannoproteins and a-(1-3)-glucan. The least studied are α-glucans, which are a linear polymer of glucose (in Schizosaccharomyces pombe, such a glucan contains approximately 260 glucose residues). This polymer consists of two linked linear chains containing about 120 (1-3)-a-D-glucose residues and (1-4)-a-D-glucose residues at the ends of the polymer molecule. This glucan is thought to be essential for the process of morphogenesis. A water-insoluble glucan was isolated from the CS of the Penicillium roqueforti mycelium, which, after drying, loses the ability to dissolve in alkali, but it was successfully dissolved in a 10% solution of lithium chloride in dimethyl sulfoxide. In the study of glucan by the methylation method, two, tri-O-methyl-derivatives of glucose were obtained, corresponding to bonds 1 - 3 and 1 -» - 4 between monosaccharides in a ratio of about 5: 2, and traces of tetra-O-methyl-derivative, corresponding to terminal non-reducing monosaccharide residues. This result indicates a linear structure of molecules containing only 1 -»- 3 and 1-»- 4 bonds between glucose residues. These data were confirmed by analysis of the 13C-NMR spectrum of the polysaccharide, which also shows the a-configuration of all glucose residues. Such polysaccharides, especially after chemical modification (sulfation or carboxymethylation), which can make them soluble in water, can be used as biologically active polymers and are of interest for studying their biological function in the cell wall of fungi and plants.
Water-insoluble (1-»-3)-a^-glucans were previously isolated from several species of higher and lower fungi, including Penicillium chrysogenum, and related a^-glucans with two types of bonds (1-3) and (1 - "- 4), differing in their ratio, were found in higher plants (Aconitum kusnezoffii Reichb).
The structural component - cellulose - is characteristic of oomycete fungi, which, according to modern taxonomy, are classified as pseudofungi. The monosaccharide composition of CS includes glucose, mannose, xylose, and the predominant sugar is glucose, which makes up to 68%. The composition of fungal CS also includes amino acids, lipids (not more than 3%) and ^-acetyl-^-glucosamine. Glucans, such as 1,3-glucan, form a strong complex with chitin called the chitin-glucan complex (CGC), which makes up the skeleton of the fungal cell. This complex is present in the SC in almost all fungi, with the exception of Zygomycetes, which is a reliable systematic feature. In some fungi, on the surface of the SC, a mucous material was found, consisting of polysaccharides represented by ß-1,3-glucans containing only glucose units connected by ß-1,6-bonds with every third unit of glucose
prokaryotic cell
prokaryotes- organisms that, unlike eukaryotes, do not have a formed cell nucleus and other internal membrane organelles (with the exception of flat cisterns in photosynthetic species, for example, in cyanobacteria). The only large circular (in some species - linear) double-stranded DNA molecule, which contains the main part of the genetic material of the cell (the so-called nucleoid) does not form a complex with histone proteins (the so-called chromatin). Prokaryotes include bacteria, including cyanobacteria (blue-green algae), and archaea. The descendants of prokaryotic cells are the organelles of eukaryotic cells - mitochondria and plastids. The main content of the cell, which fills its entire volume, is a viscous granular cytoplasm.
eukaryotes- organisms that, unlike prokaryotes, have a well-shaped cell nucleus, delimited from the cytoplasm by the nuclear membrane. The genetic material is enclosed in several linear double-stranded DNA molecules (depending on the type of organisms, their number per nucleus can vary from two to several hundred), attached from the inside to the membrane of the cell nucleus and forming in the vast majority (except dinoflagellates) a complex with histone proteins, called chromatin. Eukaryotic cells have a system of internal membranes that form, in addition to the nucleus, a number of other organelles (endoplasmic reticulum, Golgi apparatus, etc.). In addition, the vast majority have permanent intracellular symbionts-prokaryotes - mitochondria, and algae and plants also have plastids.
2) cytoplasm
Cytoplasm- the internal environment of a living or dead cell, except for the nucleus and vacuole, limited by the plasma membrane. It includes hyaloplasm - the main transparent substance of the cytoplasm, the obligatory cellular components in it - organelles, as well as various non-permanent structures - inclusions.
The cytoplasm contains all kinds of organic and inorganic substances. It also contains insoluble waste products of metabolic processes and reserve nutrients. The main substance of the cytoplasm is water.
The cytoplasm is constantly moving, flowing inside a living cell, moving with it various substances, inclusions and organelles. This movement is called cyclosis. All metabolic processes take place in it.
The cytoplasm is capable of growth and reproduction and, if partially removed, can be restored. However, the cytoplasm functions normally only in the presence of a nucleus. Without it, the cytoplasm cannot exist for a long time, just like the nucleus without cytoplasm.
The most important role of the cytoplasm is to unite all cellular structures (components) and ensure their chemical interaction. The cytoplasm also maintains the turgor (volume) of the cell, maintaining the temperature.
3) Cell wall
cell wall- a rigid shell of the cell, located outside the cytoplasmic membrane and performing structural, protective and transport functions. Found in most bacteria, archaea, fungi and plants. Animals and many protozoa do not have a cell wall.
Cell walls of prokaryotes
Bacterial cell walls are composed of peptidoglycan (murein) and come in two types: Gram-positive and Gram-negative. The cell wall of the gram-positive type consists exclusively of a thick layer of peptidoglycan, tightly adjacent to the cell membrane and permeated with teichoic or poteichoic acids. In the gram-negative type, the peptidoglycan layer is much thinner, there is a periplasmic space between it and the plasma membrane, and outside the cell is surrounded by another membrane, represented by the so-called. lipopolysaccharide and is a pyrogenic endotoxin of Gram-negative bacteria.
Fungal cell walls
The cell walls of fungi are composed of chitin and glucans.
Actinomycetes (Actinomycetales, from Greek aktis - ray, mykes - mushroom) are branching bacteria belonging to the Actinobacteria phylum. They are part of the normal microflora of the digestive system of terrestrial vertebrates and invertebrates, and are also abundant in the soil and play an important role in the ecology and cycle of substances in the soil.
These microorganisms are the causative agents of many opportunistic pathologies, such as those that result from a decrease in the function of the body's immune system. Actinomycetes are widely used in biotechnology, as they are a source of a number of antibacterial and antitumor substances.
Rice. 1. Streptomycetes synthesize a huge amount of antibacterial and antitumor drugs.
The structure of actinomycetes: why are bacteria and not fungi?
1. Organization of genetic material
The hereditary material of Actinomycetes is enclosed in a single molecule of deoxyribonucleic acid, which has a ring shape and is freely located in the cytoplasm - the same form of organization of the genetic material, called the nucleoid, is also characteristic of other bacteria. In fungi, the genetic material is organized and is part of the cell nucleus.
The DNA of actinomycetes contains a large number of GC pairs (65-75% of the total number of nucleotides). This feature is constant, does not depend on mutations, and therefore is used in the taxonomy of microorganisms. Such a content of GC pairs makes the DNA of actinomycetes very refractory, therefore, the analysis of DNA of actinomycetes takes more time compared to other bacteria.

Rice. 2. Schematic structure of the cell wall of Gr+ bacteria.

Rice. 3. Actinomycetes stained by Gram.
Actinomycetes have a dense bacterial cell wall, which is located outside the cytoplasmic membrane and causes their positive Gram staining. Like other Gram-positive bacteria, it consists of several dozen layers of murein (peptidoglycan) polymer, which is permeated with teichoic and lipoteichoic acids. Lipoteichoic acids are anchored in the cytoplasmic membrane of bacteria and connect it to the cell wall. Teichoic acids give the cell wall a negative charge. The cell wall of fungi, on the other hand, consists of other polymers - chitin and glucan.
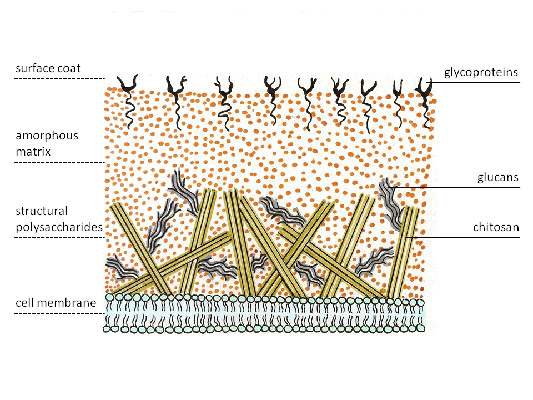
Rice. 4. Cell wall of fungi.
3. Cell organelles
Actinomycetes, like other bacteria, do not have membrane organelles. Actinomycetes have 70S ribosomes, while fungi have 80S ribosomes, as do other eukaryotic organisms.
4. Growth of colonies
The formation of mycelium during growth is what most actinomycetes have in common with fungi. Mycelium in the case of actinomycetes is a branching collection of hyphae. The hyphae are separated by septa into long bacterial cells containing several nucleoids. Partitions in a number of species can pass in a mutually perpendicular direction. Hyphae branch by budding.
Mycelium growing into the substrate (earth, silt or nutrient medium) is called the substrate. It provides the colony with nutrients. Aerial mycelium rises above the substrate, giving the colony "fluffiness" - it forms spores, as well as the so-called "secondary metabolites" (in contrast to the "primary metabolites" of the substrate mycelium), among which there are many antibacterial substances.
Life cycle and physiology of actinomycetes
In progress life cycle most of actinomycetes produce spores. Some actinomycetes reproduce by fragmenting the mycelium.
1. Sporulation
Spores of actinomycetes originate from aerial mycelium. These are exospores - they develop outside the mother cell. The hyphae of the aerial mycelium, from which spores develop, are called spore bearers. Spores can be contained in a thickening at the end of the spore-bearing sporangium (for example, in streptomycetes, actinoplans and plymelia), or they can be located in a chain along the sporangiophore (for example, in nocardia and actinomadura).
According to the number of spores formed, actinomycetes are divided into:
- Monospores (for example, Saccaromonospora, Micromonospora, Thermomonospora) - form single spores, more often by budding and subsequent separation by a septum from the parent hyphae;
- Oligospores (for example, Actinomadura) - form short chains of spores along the spore-bearing;
- Polyspores (most other actinomycetes, for example, Streptomyces, Frankia, Geodermatophilus) - form many spores enclosed in sporangia.
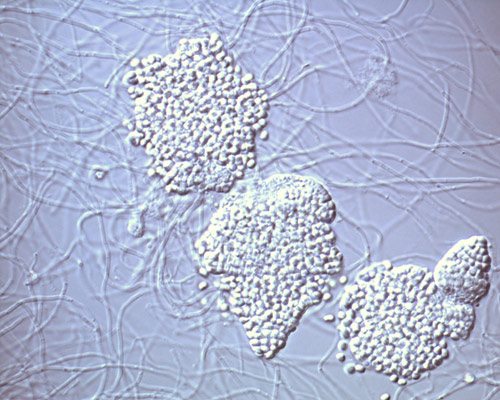
Rice. 5. Sporangium of actinomycetes of the genus Frankia.
Spores of actinomycetes can be mobile - in this case, the spore has a flagellum and can move (spores of actinoplanes, geodermatophiles and dermatophiles). In most cases, the spores are immobile and spread by wind, water, or animals.

Rice. 6. Dermatophiles, light microscopy.
Sporulation in actinomycetes is especially active under unfavorable conditions. The resistance of spores to heat is low compared to spores of other bacteria, but they withstand drying no worse than others, and therefore have tremendous adaptive significance. Actinomycetes dominate other microorganisms in dry desert soils.
The germination of the pack requires a certain humidity of the external environment. In the presence of water, the spore swells, enzymes are activated in it, and metabolic processes are triggered, accompanied by the release of growth tubes (future bacterial bodies) and the synthesis of nucleic acids.
2. Type of breathing
Most actinomycetes are aerobes (need oxygen to sustain life). Facultative anaerobes (bacteria that can live both in the presence and in the absence of oxygen) are found among species with a short mycelial stage, reproducing by fragmentation of the mycelium.
3.Acid resistance
Actinomycetes are acid tolerant, resistant to an acidic environment, which allows them to live in acid-saturated forest soils. Acid resistance in the laboratory can be determined by Ziehl-Neelsen staining of a preparation containing actinomycetes (magenta followed by etching with sulfuric acid and staining with methylene blue). Most actinomycetes with this color do not discolor after pickling with acid and retain a red magenta color. The alkaline environment is unfavorable for these bacteria: at elevated pH, they are prone to sporulation.
4. Features of metabolism
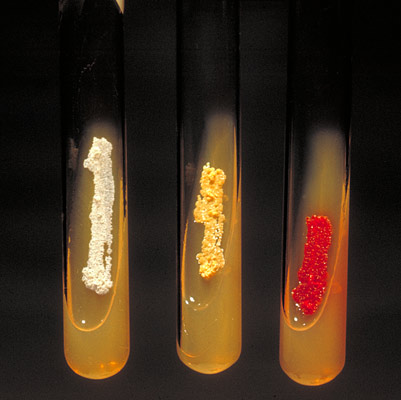
Rice. 7. Aerobic actinomycetes form pigment on agar slant. From left to right: Actinomadura madurae, Nocardia asteroides, Micromonospora.
The formation of "secondary metabolites" by aerial mycelium was mentioned above. Among them:
- pigments that cause different colors of aerial mycelium during growth on media;
- volatile odorous substances that give a characteristic smell to the soil after rain, stagnant water, the skin of some animals;
- antibiotics:
a. antifungal - polyenes;
b. antibacterial - for example, streptomycin, erythromycin, tetracycline, vancomycin;
c. antitumor - anthracyclines, bleomycin.
Where do actinomycetes live?
Actinomycetes in most are found in soils, moreover, mycelial forms are much less than spores. They play a significant role in the formation of humus, decomposing organic matter, difficult to be utilized by other bacteria. In this regard, actinomycetes are used as sanitary indicative microorganisms in sanitary and epidemiological matters: their detection in large quantities in soil or water indicates the presence of compost in the corresponding substrate.

Rice. 8. Actinomycetes in compost.
Actinomycetes are symbionts of many plants, helping them to fix nitrogen. At the same time, many microorganisms of this class are causative agents of plant diseases.

Rice. 9. Potato streptomycosis.
They are also found in the normal microflora of the digestive system of a range of animals, ranging from soil annelids (such as earthworms) to large livestock.
These microorganisms help break down cellulose, which is abundant in plant foods. In humans, actinomycetes are found in the oral cavity (gums and plaque), intestines (distal large intestine), on the skin (face, wings of the nose, behind the ears, between the fingers) and in the organs of the respiratory system (mainly in the upper respiratory tract).
![]()
Rice. 10. Microflora of human skin. The Actinobacteria phylum is indicated in shades of blue, the Actinomycetes class in bright blue.
Actinomycetes, subject to a decrease in the body's immune reactivity, can cause actinomycosis - opportunistic diseases, consisting in the formation of actinomycosis granulomas - clusters of bacterial bodies resembling grains of yellow sulfur ("druze"), surrounded by immunocompetent cells. The inflammatory reaction leads to granuloma melting, fistula formation leading to organ perforations and spread of bacteria by blood.
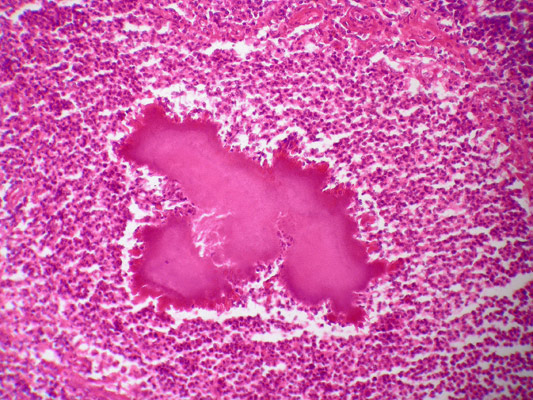
Rice. 11. Actinomycotic drusen, Gram stain.
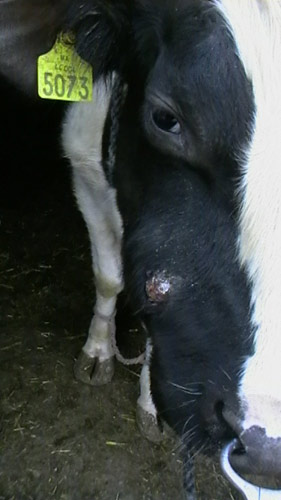
Rice. 12. Actinomycosis of the upper jaw in a cow.

Rice. 13. Human maxillary actinomycosis.
Actinomycetes are amazing organisms that still mislead many scientists with their similarity to fungi. Along with the potential danger in the form of opportunistic actinomycosis, these organisms give humans fertile soil and weapons to fight infectious and oncological diseases - antibiotics and cytostatics.
