VACUOLES. SUBSTANCES OF CELL JUICE
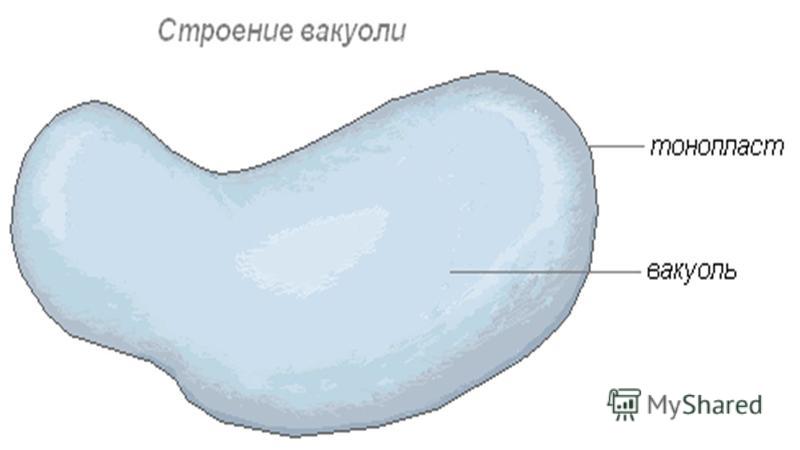
A vacuole is a cavity within the cytoplasm bounded by a membrane called tonoplast , and containing cell sap. Vacuoles are formed with the participation of vesicles of the Golgi apparatus and by separation of vesicles from the agranular (not containing ribosomes) EPS.
Young plant cells have small vacuoles. In old plant cells there are usually several large vacuoles with cell sap or one that occupies almost the entire internal space of the cell. When large vacuoles appear, the protoplast in the form of a thin peripheral layer is pressed against the cell wall.
Functions of vacuoles:
1. water absorption and turgor maintenance plays an important role in the elongation of cells during their growth and in the general water regime of the plant;
2. accumulation of metabolic products plants (reserve and excretory);
3. presence of pigments in cell sap determines color flowers, fruits, leaves, etc. attracts pollinators and seed and fruit dispersers;
4. destruction of macromolecules, circulation of their components in the cell. Individual components of the cell can enter the vacuole and be destroyed there. By this digestive activity of the vacuole compared to lysosomes.
Composition of cell sap
The main component of cell sap is water. The remaining substances vary depending on the type of plant and its physiological state. Often the concentration of substances in cell sap can be higher than in the surrounding cytoplasm. In this case, vacuoles can form crystals. Cell sap is usually has an acidic, rarely neutral and even less often alkaline reaction ( for example, cucumber, pumpkin, melon). The main groups of cell sap substances:
I. Organic acids or their acid salts cause an acidic reaction in cell sap. Most often found in the cell sap of unripe fruits. When the fruits ripen they are used as a substrate for respiration (in the Krebs cycle), so the sour taste disappears. These include:
1. Oxalic acid predominates in sorrel leaves, etc. A lot of oxalic acid is extracted from peat.
2. Malic acid predominates in the fruits of apple, rowan, cherry, and tomato. The most sour variety of apples, Antonovka, contains about 0.5% malic acid.
3. Citric acid predominates in the fruits of lemon, cranberry, red currant, and tobacco leaves. Lemon fruit juice contains about 6% citric acid, tobacco leaves contain 8-14%. Citric acid is extracted mainly from tobacco leaves. When blood is transfused to a person, sodium citric acid is used to preserve it.
4. Tartaric acid predominates in grapes and raspberries. Grape juice contains about 0.3% tartaric acid.
5. Found in cell sap amino acids and other organic acids.
II. Carbohydrates, cause the sweet taste of cell sap. Carbohydrates in cell sap play a role reserve nutrients. Among them:
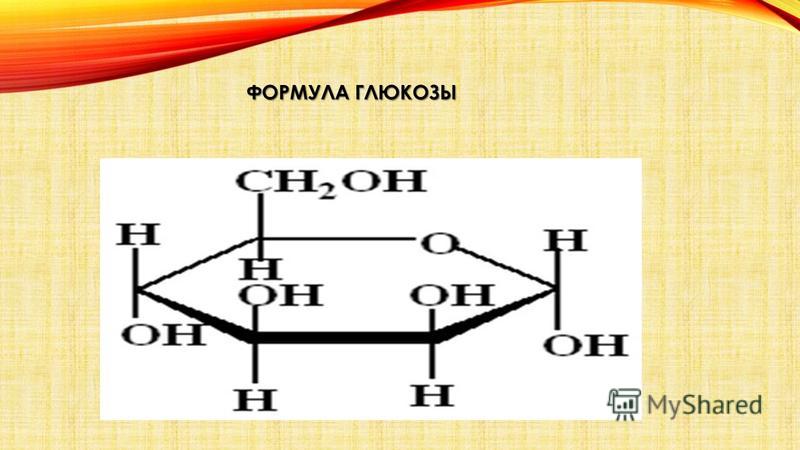
1. Glucose, or grape sugar, is the simplest carbohydrate. Most often found in plant fruits and included in honey. Glucose is a solid, colorless, crystallizing substance. Easily dissolves in water. It is a simple sugar, or monosaccharide. In a plant, with the help of enzymes, glucose is often converted into starch and vice versa. Insoluble, formed during photosynthesis from glucose primary starch turns into glucose, which easily dissolves in cell sap, passes through the cell membrane and is transferred from leaves to fruits or other parts of the plant, where it turns into secondary starch . Glucose is a source of energy in the body. It is widely used in medicine.
2. Fructose, or fruit sugar, predominates in ripe fruits, is included in the composition of honey. As As fruits ripen, glucose transforms into its isomer, the sweeter fructose, and the fruits become sweeter. Fructose is a solid, colorless, easily soluble monosaccharide.
3. Sucrose predominates in the cell sap of root vegetables sugar beets, stems sugar cane, fruits of watermelon, melon, etc. Sugar used in food is sucrose. The roots of sugar beets contain up to 26%, and the stems of sugar cane - up to 20% sucrose.
The Canadian flag features a leaf sugar maple. In those days when sugar cane was not yet known in America, sugar maple was the most important source of sugar. Its sweet sap was used to make maple syrups, molasses, and even maple beer. The maple leaf has become a symbol of this country.
From palm sap(palmyra, sugar palm, mangrove palm, etc.) provide the basis for the production of sugar, wine, alcohol and vinegar.
4. Inulin found dissolved in the cell sap of many plants, but predominates in plants family Asteraceae, where it replaces starch (for example, in dahlia tubers, Jerusalem artichoke (earthen pear), in roots). Inulin - starch isomer, colorless substance. Inulin is not stained by iodine and does not form a paste. Inulin spherocrystals sometimes grow so large that they occupy the space of several cells. The monomer of inulin is fructose, and not glucose as for most polysaccharides, which allows the use of products containing inulin in dietary nutrition of diabetic patients.
5. Pectin substances often found dissolved in cell sap. The fruits of apple, quince, plum, etc. are rich in pectin substances. Pectin substances are used in confectionery industry as giving jelly(jelly, marmalade, pastille, etc.). These substances are of great dietary importance for humans.
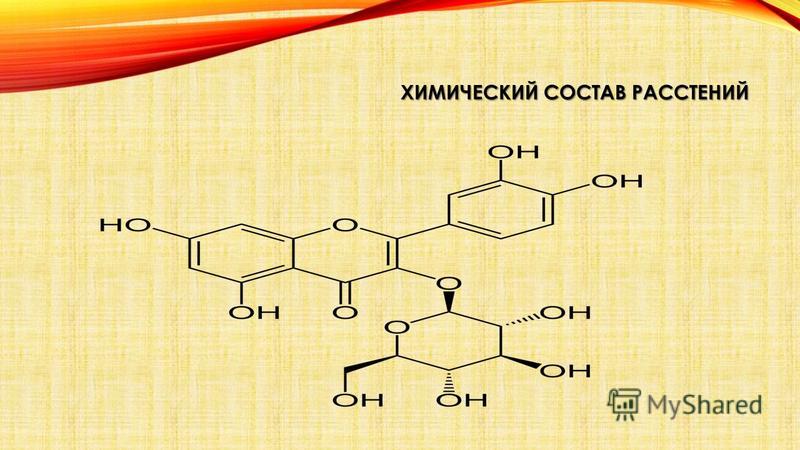
III. Glycosides - these are complex organic matter, compounds of glucose with alcohols, aldehydes and other substances. Many glycosides poisonous. Many are used in medicine, technology and in everyday life. Nice the smell of tea, coffee, vanilla, mustard is caused by glycosides that decompose upon contact with air under the influence of enzymes and release volatile substances.
The seeds of bitter almonds, apricots, peach, plums, cherries, pears, and apples contain a glycoside amygdalin and enzymes that decompose amygdalin into glucose, benzoaldehyde, which smells like almonds, and hydrocyanic acid, which is a powerful poison.
Lily of the valley flowers and foxglove leaves contain cardiac glycosides, acting on the activity of the heart and therefore used in medicine.
Many plant pigments also belong to the group of glycosides. The most common pigments are anthocyanins and flavones.
Anthocyanins observed in flower petals, leaves, stems, fruits and seeds. They are red, purple or Blue colour depending on the reaction of the cell sap:
· if the cell sap is acidic, anthocyanins are red in color,
· if neutral - purple,
· if alkaline - blue.
Since the reaction of the juice can change in the same cells, their color also changes. So, the lungwort plant has red flowers at the beginning of flowering, then their color becomes purple and, finally, at the end of flowering - blue.
Black color, for example, grape fruits, black nightshade, etc., is explained by the fact that dark purple anthocyanins are present not only in the skin cells, but also in the fruit pulp cells.
Flavones - yellow or orange pigments. The yellow and orange fruits of tangerines, lemons, oranges, and the yellow flowers of some plants are colored with it.
So, the color of plants depends on the pigments dissolved in cell sap (anthocyanins, flavones, etc..), and from pigments formed in plastids (chlorophyll, xanthophyll, carotene, etc.). It is very easy to find out what pigments the color depends on. It is enough to boil the part of the plant in question in water: if the pigments were in the plastids, they will not transfer into the water, which will remain colorless, and the part of the plant will retain its previous color (for example, carrot root in soup); if the pigments were in the cell sap, they will go into the water, since the cytoplasm, killed by high temperature, will freely pass through the cell sap and the water will become colored, and part of the plant will turn pale or completely discolored (for example, the root of red beetroot in borscht).
Biological significance pigments are very large. The bright colors of flowers and fruits attract flower pollinators and fruit and seed dispersers.
Also classified as glacosides are those with a sweet taste. stevioside (300 times sweeter than sucrose!), extracted from the leaves of a plant stevia. Stevioside is registered in the food industry as food additive E960 as a sweetener, 25 kg of stevioside is equivalent to 5 tons of sugar. Widely used in the Japanese food industry.
IV. Alkaloids widespread in flora. Alkaloids are usually named after the Latin name of the genus or species of plant in which they are found. Thus, the name of the tobacco alkaloid nicotine is from Nicotiana, the poppy alkaloid papaverine is from Papaver . Alkaloids - complex organic, nitrogen-containing substances, bitter and in most cases very poisonous.
Alkaloids are widely used in everyday life and in medicine. Here are examples of some of them:
Thus, tea leaves and coffee tree seeds contain an alkaloid caffeine, tobacco leaves - nicotine. These alkaloids, as acting on central nervous system used in everyday life in the form of tea, coffee, and smoking tobacco.
Alkaloid quinine, extracted from the bark of the cinchona tree, saved humanity from malaria.
Alkaloids morphine, codeine, papaverine and others (22 in total) are contained in the milky juice of poppy seeds, called opium. Morphine - painkiller and sleeping pills, papaverine - painkiller, codeine - cough sedative.
Alkaloid cocaine, extracted from the leaves of the South American coca bush, - a means for local anesthesia(pain relief).
Alkaloid strychnine extracted from seeds chilibukhs(Strichnos) - central nervous system stimulant.
Alkaloid atropine, extracted from the leaves and seeds of belladonna and datura and henbane leaves is used for some gastric diseases and in eye practice as a dilating pupil.
Many plants are poisonous because they contain poisonous alkaloids (often along with other poisonous substances). Yes, a poisonous mushroom fly agaric contains poisonous alkaloids muscarine and amanitotoxin.
Young stems and skins of potato tubers contain a poisonous alkaloid. solanine, which disintegrates when boiled in water.
In beans cocoa fruit(Theobroma – translated from Greek as “food of the gods”) contains theobromine, causing a surge of strength, improving mood, dilating blood vessels, but not providing harmful effects on the central nervous system, like coffee. Cocoa grains are used to make chocolate bars, candies, cocoa drink, and the oil squeezed from the grains is used in perfumery to make creams and lipsticks.
V. Tannins, or tannins - these are organic substances, similar in composition to glycosides, giving vacuoles yellow-green color . A characteristic property of these substances is their astringent taste and acid reaction. Tannins are used in medicine for inflammation of the mucous membranes. Many tannins are found in the bark of eucalyptus, oak, willow, and tea leaves. Richest in tannins galls (ink nuts) - painful growths, formed on leaves due to the deposition of eggs in the leaf tissue by insects.
Tannins are even more important for the leather industry. Combining with leather proteins, they produce insoluble sediments, which are used for tanning leather. Due to tanning the skin becomes soft, does not allow water to pass through and does not become slimy.
VII. Inorganic substances in cell sap they are represented by salts of nitric, phosphoric and other acids. Great importance in human nutrition and treatment they contain salts of magnesium, potassium, calcium, iron, etc., for example:
· magnesium rich in potatoes, cabbage, tomatoes, apricots, peaches.
· potassium- potatoes, cabbage, tomatoes, apricots, peaches, eggplants.
· a lot of glands a, which plays a large role in hematopoiesis, in oxidative processes and growth processes, contains: strawberries, melons, mushrooms, wheat and rice (bran), beets, cucumbers, lettuce, onions, tomatoes, legumes.
As a result of metabolism in plant cell vacuoles are formed - spaces filled with solution various substances- waste products of protoplast. This solution is cell sap. In young cells there is little cell sap and the vacuoles look like very small bubbles of a viscous colloidal nature, but as the cell grows they liquefy, enlarge, and merge with each other. Eventually, one large vacuole is formed in the cell, and the cytoplasm encloses it in a thin layer and is located wall-to-wall. The vacuole is separated from the cytoplasm by the tonoplast.
Chemical composition of cell sap varies greatly depending on the type of plant. A huge variety of chemicals isolated from plants and having medicinal properties are found in cell sap. Cell sap contains 2 groups of substances: products of primary metabolism necessary for the life of plants (proteins, fats, carbohydrates) and substances of secondary metabolism (alkaloids, glycosides, tannins, etc.). Cell sap often has an acidic reaction.
Products of primary exchange: carbohydrates (mono- and disaccharides - glucose, fructose, sucrose), simple soluble proteins, fats in the form of glycerol and fatty acids.
Secondary products:
Glycosides cell sap - compounds of certain sugars (usually glucose) with alcohols, aldehydes, phenols and other organic substances. When exposed to air under the influence of enzymes, they quickly disintegrate, often releasing a pleasant odor. This explains the smells of tea, coffee, cocoa, tobacco, mustard, and vanilla. Glycosides include the following substances: amygdalin (in almond and apricot seeds); saponins used as detergents (soapwort); coumarins - in sweet clover leaves, etc.; cardiac glycosides - in foxglove leaves.
Tannins(tannins) are complex organic nitrogen-free compounds with an astringent taste. They have antiseptic properties, which protects plants from damage by microorganisms. Widely distributed in the plant world: in oak bark 10-20%, in tea leaves 15-20%, willow bark 9-13%, in the fruits of persimmon, quince, dogwood. They are used in medicine as an astringent, in the textile industry for dyeing fabrics dark brown, and in the tanning industry for tanning leather.
Alkaloids- nitrogenous salts of organic acids: malic, tartaric, etc. are insoluble in alkalis, soluble in water. Formed in all parts of the plant: in the roots and leaves of belladonna (atropine), in the seeds and milky juice of poppy - papaverine, morphine, codeine; tobacco leaves contain nicotine; in potato tubers - solanine; in fly agaric - muscarine.
Widely used in medicine and agriculture; in small doses they have a stimulating effect on the nerve centers, in large doses they have a paralyzing effect. Quinine - against malaria, strychnine - stimulates muscle activity; cocaine is an analgesic; morphine is an analgesic and sleeping pill; papaverine - vasodilator; nicotine - used in agriculture to control insects. Plants containing alkaloids are poisonous and are not eaten by animals. In cells containing alkaloids, spores and germs of microorganisms do not develop, plants are not affected by fungal and bacterial flora (protective role).
Cell sap is rich in various organic acids: malic, tartaric, oxalic, citric, amber, etc. Functions are varied: they participate in the respiration process, partly act as phytoncides and antibiotics, protecting the plant from damage by fungi, viral, bacterial diseases, providing plant taste and smell due to volatile acids: formic , oil, vinegar.
Substances related to carbohydrates are widespread in cell sap - pectins. Easily gelatinous, used in the confectionery industry. In pharmacy - for the preparation of a number of dosage forms (as an emulsifier in emulsions, in pills - as a binding component).
Various coloring substances accumulate in the cell sap - pigments, specific for each plant species. Anthocyanins are the most common and are present in the form of glycosides. Depending on the reactions that it undergoes in the cell sap with salts, tannins, and acids, it gives different colors to the cell sap. The extraordinary variety of colors of flowers in plants, as well as leaves, is most often associated with anthocyanins. Red poppies, red clover heads, blue and blue hyacinths, blue cornflowers - all this is created by anthocyanin. Anthocyanin should not be mixed with chlorophyll, carotene, xanthophyll and other plastid pigments. In addition to attracting insects, anthocyanin has a protective effect against low temperatures and harmful short-wavelength waves. Of the yellow pigments, anthochlor is found in cell sap (in the flowers of yellow poppy, mullein, toadflax, and in citrus fruits).
Essential for cell life are vitamins. This group of organic compounds of diverse chemical nature, plant, and less often animal origin, is closely related to enzymes; grouped based on their physiological effects on the body. They are divided into two groups: water-soluble (C, B) and fat-soluble (A, D, E). The highest content of vitamins is observed in leaves, ripened fruits, and roots. Some (E) are contained in the embryos of seeds, others (D) - in germinating seeds.
Minimum doses are necessary for the normal functioning of the plants themselves (to maintain growth, regulate respiration, metabolism, etc.).
The effect on animals and humans is known.
For plants, B 1 - for the development of the root system, B 2 - is involved in respiration. Contained in the peel and germs of seeds, in live yeast, in the sprouts of wheat, barley, rice, and in rice bran.
Vitamin C - ascorbic acid - determines the oxidative activity of enzymes and therefore regulates the respiration process.
Vitamin A is formed in the liver from provitamin A, which is a yellow pigment - carotene - found in plastids. The yellow color of plant sprouts, corn grains, and carrots is provided by the presence of provitamin A.
Provitamin D (ergosterol) is found in plants, vegetable oils, and sawdust. Under the influence of sunlight, vitamin D is formed in the germ layer of the epidermis, regulates the exchange of calcium and phosphorus and their ratio in the blood and bone matter.
Vitamin E - affects the sexual sphere (found in unrefined cottonseed oil, soybean oil, corn oil, citrus fruits, tomatoes)
Vitamin PP - nicotinic acid - in yeast and rice bran - causes painful changes in the skin, digestive tract, and nervous system. Catalyzes redox reactions and actively participates in carbohydrate metabolism.
Vitamin K - ensures blood clotting (spinach, alfalfa, cabbage, nettle).
Some plant organs are especially rich in vitamins - green leaves, stems, fruits of berries, fruits and vegetables. For example: nettle leaves, red pepper fruits, rose hips contain several vitamins at once - A, B, C, K and others.
The protoplast of a plant cell also produces a special group of substances that have the property of enhancing physiological processes. Such substances are called phytohormones. Phytohormones have been identified that enhance growth, cell division, and sexual functions.
Growth hormones - auxins increase the access of oxygen, the flow of nutrients to embryonic tissues and create conditions for growth processes. The chemical composition of auxins was studied, first isolated and then synthesized artificial heteroauxin, which increases the yield of cucumbers, tomatoes, peppers, hemp and other industrial and vegetable crops.
The first antibiotic, penicillin, isolated in 1928 by Flemming from the mold Penicillum sp., revolutionized the treatment of infectious diseases in the Second World War. Later streptomycin and other antibiotics were isolated.
Phytoncides were discovered in flowering plants by B.P. Tokin. Their chemical composition is varied: these are alkaloids (onion, mustard), organic acids (oxalic, malic, tartaric, succinic), essential oil (garlic). Many types of mono- and dicotyledonous plants: yarrow, wormwood, bird cherry, birch, onion, garlic, etc.
Entry of substances into the plant cell. The vital activity of the body, all organs and cells is possible only with metabolic processes continuously occurring in them. The cell absorbs substances from the environment and at the same time transfers the products formed in it to neighboring cells or releases them into the external environment.
The ability of the protoplast to continuously exchange with the environment bears the features of selectivity. Of the large number of substances located outside the cell, under normal conditions only certain compounds penetrate into it in certain proportions. Accordingly, only certain waste products are released by the cell into the environment. In the phenomena of absorption and release of substances by a cell, the processes of diffusion and osmosis play an important role. As is known, the particles of the substances that make up protoplasm have a certain cellular energy, which is the reason for their continuous movement. The movement of dispersed matter from one part of a system to another is called diffusion. This is not a chaotic movement of molecules, but a directional one, the nature of which is determined by a number of factors: the activity of diffusing molecules, the gradient of concentrated solutions; the rate of diffusion is determined by the size and mass of the molecules, the viscosity of the medium, temperature, composition and properties of other compounds in the solution, and other conditions. The complexity and heterogeneity of the structure of protoplasm determines the unequal rate of diffusion in various parts one cell. If the diffusing substance encounters a membrane with different permeability for the solvent and solute on its way, the movement of substances in such a system becomes more difficult. Being an obstacle to the free diffusion of electrolytes, it ensures a constant difference in concentration between the cell sap and the solution surrounding the cell. The penetration of liquid and soluble substances through semi-permeable partitions is called osmosis. The phenomena of adsorption and desorption are of primary importance in the process of osmosis. They are accompanied by electroosmotic processes. Osmotic pressure in a cell does not depend on protoplast colloids, but on solutions of various salts, sugars, and amino acids in cell sap. For any dissolved salts to penetrate into the cell from the outside, it is necessary that the osmotic pressure of the cell sap be higher than in the saline solution surrounding the cell. Salts (electrolytes) enter the cell not in the form of molecules, but as individual ions that are adsorbed on the surface of semipermeable membranes due to its electric potential. Ions also have their own charges, and the larger they are, the more difficult it is for them to penetrate into the cell. The adsorbed ions are then desorbed onto the inner wall of the plasmalemma and transferred to the mesoplasm. Sorption processes are of an exchange nature. The intensity of these phenomena depends on the respiration of cells. The energy released during the stepwise breakdown of substances during respiration is used to a large extent for the sorption functions of cells.
If living cell put into a highly dilute aqueous solution of saltpeter, an osmotic interaction immediately begins between the cell sap and the surrounding solution. Cell sap, which is a solution of different substances in different concentrations, will have a higher osmotic pressure than the external solution and will attract water from it. Cell sap, having increased in volume, will put pressure on the cytoplasm, the latter on the cell membrane, stretching it in all directions. Possessing elasticity, the shell will resist the pressure of cell sap. Since the shell has limited elongation, the resistance will increase as the pressure from the addition of water increases. At a certain moment, this resistance force will balance the osmotic pressure, although the concentration of both solutions will not yet be homogeneous. The state of tension in the cell membrane is called turgor, and the pressure is turgor.
The degree of turgor depends on the difference in osmotic pressure inside and outside the cell and on the elasticity of the membrane. The combined turgor of the mass of cells in the plant’s body creates tension and elasticity of the entire plant, helps the stems maintain an upright position, maintain the mass of leaves, withstand wind, storms, showers, and orient the leaves in relation to the light. In a word, turgor ensures the normal physiological state of the plant.
The difference in osmotic pressure inside and outside the cell provides the suction force of the cells.
The opposite of turgor occurs if the cell is placed in a strong solution of table salt, more concentrated than cell sap. In this case, compression of the shell and protoplast will begin, but since the membrane is less elastic, its compression will soon stop, while the cytoplasm, continuing to contract, will move away from the cell wall and take the form of a lump inside the cell. This phenomenon is called plasmolysis. Plasmolysis in plant tissues makes them sluggish, organs become flabby. It can be curved (protoplast is round); concave (the protoplast in some places does not tear away from the shell, but is partially pulled inward); convulsive (without a specific pattern). If you place a plasmolysis cell in clean water, a phenomenon opposite to plasmolysis is observed - deplasmolysis.
Under certain conditions, with the loss of cell turgor, cytorhiz is observed, when the entire cell (with its membrane) contracts. It is observed when plants wilt and is not a consequence of water loss by osmosis, but the result of water evaporation.
All chemical substances Depending on their functions, cells can be divided into three groups: constitutional, reserve, excretory.
Constitutional substances participate in the construction of the cell body: all its parts and organelles. These are complex (building) proteins that make up membranes, hyaloplasm, karyoplasm, ribosomes, proteins - histones (chromatin threads), etc. Carbohydrates in the form of polysaccharides are part of the shell; Lipids (phospholipids) – are part of membranes.
Spare substances There are soluble (extractive) and insoluble (actually spare). Extractives: soluble proteins, carbohydrates - glucose, sucrose, fructose and fats in the form of glycerol and fatty acids are found mainly in cell sap and partly in the cytoplasm of cells of various tissues. Insoluble deposited in storage tissue. Carbohydrates are stored in the form of starch grains formed from amyloplasts in storage organs (rhizomes, tubers, bulbs, endosperm, etc.). From a physiological point of view, assimilation, transitory and storage starch are distinguished. Assimilation is formed in chloroplasts during photosynthesis from excess glucose, thereby normalizing osmotic pressure. Along the way from the assimilation organs to the storage organs, glucose is also partially converted into starch (in sieve tubes), called transient. It can be saccharified again and continue to move into storage tissues, where it is deposited in the form of starch grains. Starch grains are formed from amyloplasts and in structure are spherocrystals of the finest radially arranged needles, concentrated in layers around the so-called center of layering. The complexity of starch grains is due to the uneven supply of starch day and night. Depending on the location of the center of layering, starch grains can be eccentric (potatoes), concentric (peas, beans); simple (wheat, barley, corn), complex (oats, buckwheat), semi-complex (rye, barley). In shape - spherical, elliptical, kidney-shaped, multifaceted, in the form of a tibia, etc.; in size - small (3-10 microns for rice), large (70-100 microns for potatoes). The shape and size of starch grains is a diagnostic feature of the plant.
Starch grains contain amylose, which dissolves in hot water, stained blue by Lugol's solution, and amylopectin, which swells in hot water and turns purple. There is a so-called protected starch, which is not used by the plant even during starvation. It is deposited in the form of small starch grains in the cells of the root cap and in the endoderm.
Storage proteins are deposited as amorphous or crystalline protein (in aleurone grains). The latter are formed from protein vacuoles by dehydration. In this case, part of the protein forms a crystalloid, the other part - an amorphous body; and phytin (potassium-calcium-sodium salt of inositol hexaphosphoric acid) is a globoid. Aleurone grains can be of 3 types:
1) with globoids (seeds of legumes, cereals);
2) with globoid and crystalloid (flax and castor seeds);
3) with calcium oxalate crystals (umbelliferae, grapes).
During seed germination, the crystalloids of the aleurone grains dissolve in water, and the grains themselves merge into one central vacuole. Lugol's reagent colors aleurone grains golden yellow.
Fats are esters glycerol and fatty acids (oleic, stearic, palmitic). They are deposited in the form of droplets (sometimes in oleoplasts) in the cytoplasm, plastids, and karyoplasm. This is the most economical form of energy reserve (1g of fat - 39 kJ). They are stained orange-pink with Sudan III solution.
Excretory substances are found in cell sap. These are salts of organic and inorganic acids, most often oxalates, form various shapes crystals: rod-shaped (styloids), needle-shaped (raphides), stellate (drusy), small single (crystalline sand). The shape of the crystals is a diagnostic sign.
In some plants (nettles, mulberries), cystoliths are formed - grape-shaped outgrowths of the cell membrane inward, impregnated with calcium carbonate or silica. The formation of crystals is necessary in the cell to normalize osmotic pressure and acid-base balance; the epidermis containing crystals is shiny, as a result of which it reflects sunlight and protects plants from overheating.
Conclusion. Cell chemicals have a specific functional purpose, ensuring the vital activity of the cell and the entire plant as an integral organism.
Lecture No. 3. PLANT TISSUE - EDUCATIONAL,
End of work -
This topic belongs to the section:
Lecture No. 1. BOTANY AS A BIOLOGICAL SCIENCE. A PLANT IS A LIVING ORGANISM
Lecture BOTANY AS A BIOLOGICAL SCIENCE PLANT LIVING ORGANISM Linnaean system K... Lecture CHEMICALS... Meristems of the root growth cone...
If you need additional material on this topic, or you did not find what you were looking for, we recommend using the search in our database of works:
What will we do with the received material:
If this material was useful to you, you can save it to your page on social networks:
CELL JUICE Cell sap is an aqueous solution of various substances that are products of the vital activity of the protoplast, mainly reserve substances and waste. The reaction of cell sap is usually slightly acidic or neutral, less often alkaline. The substances that make up the cell sap are extremely diverse. These are carbohydrates, proteins, organic acids and their salts, amino acids, mineral ions, alkaloids, glycosides, tannins, pigments and other water-soluble compounds. Most of them belong to the group of ergatic substances - products of protoplast metabolism, which can appear and disappear during different periods of cell life. Many cell sap substances are formed only in plant cells.
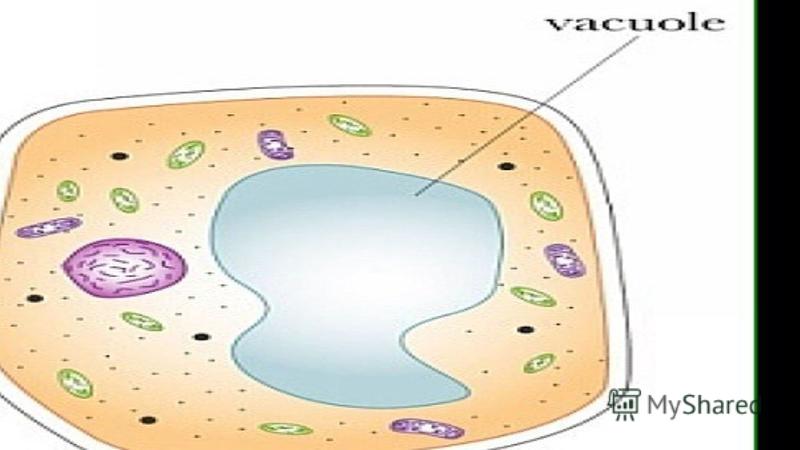
VACUOLES Vacuoles are found in almost all plant cells. They are cavities in the cell filled with watery contents - cell sap. Cell sap is isolated from the cytoplasm by a selectively permeable vacuolar membrane, the tonoplast. Tonoplast performs barrier and transport functions. Most mature plant cells are characterized by a large central vacuole, occupying up to 70-90% of the cell volume. In this case, the protoplast with all the organelles is located in the form of a very thin wall layer lining the cell wall. Small cytoplasmic vacuoles are usually found in the wall protoplast. Sometimes the nucleus is located in the center of the cell in the nuclear pocket of the cytoplasm, which is connected to the wall layer by the thinnest cytoplasmic strands crossing the central vacuole.

Glucose (grape sugar) and fructose (fruit sugar) accumulate in large quantities in juicy fruits. Sucrose (beet sugar) accumulates in large quantities in sugar beet roots and sugar cane stalks. A number of plant families (cactaceae, Crassulaceae, orchids) are characterized by the accumulation of mucus in the cell sap, which retains water. Inulin is a reserve polysaccharide, deposited as a colloidal solution in the cell sap of the underground organs of Asteraceae instead of starch. Proteins accumulate in the form of a colloidal solution in the vacuoles of cells of ripening seeds. When seeds are dehydrated in the later stages of their development, water is removed from the vacuoles, the protein concentration in the cell sap increases, and it turns into a solid gel state. Dehydrated vacuoles of mature seeds are called aleurone grains.

Of the organic acids in cell sap, the most common are citric, malic, succinic and oxalic. These acids are found in large quantities in the cell juice of unripe fruits, giving them a sour taste. When fruits ripen, organic acids can be used as respiration substrates, so the sour taste of the fruit usually disappears. Salts of organic acids, together with mineral ions, play an important role in osmotic processes. Tannids Tannins (tannins) are polymeric phenolic compounds with an astringent taste. They have antiseptic properties and protect plant tissues from infections and decay. The cells of the bark of stems and roots (oak, willow), unripe fruits (walnuts), leaves (tea) and some pathological growths - galls are especially rich in tannins. Tannids are used in medicine, for tanning leather, and dyeing fabrics dark brown. Alkaloids are chemically diverse nitrogen-containing organic substances that have a bitter taste. They have the properties of bases and are found in cell sap, usually in the form of salts. Many alkaloid-bearing plants are poisonous and are not eaten by herbivores. In cells containing alkaloids, spores and germs of microorganisms do not develop, and plants are not affected by fungal and bacterial diseases. Representatives of the families Solanaceae, Poppy, Rubiaceae, Ranunculaceae, etc. are especially rich in alkaloids.

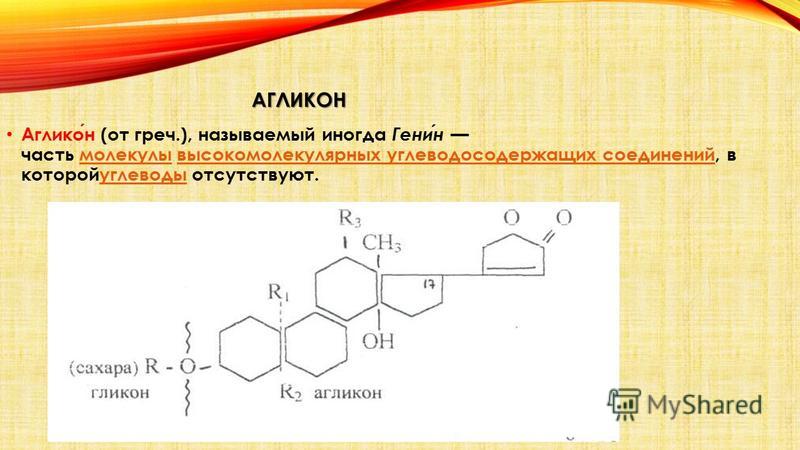
GLYCOSIDES Glycosides Glycosides organic compounds, the molecules of which consist of two parts: a carbohydrate (pyranoside or furanoside) residue and a non-carbohydrate fragment (the so-called aglycone). In a more general sense, carbohydrates consisting of two or more monosaccharide residues can also be considered as glycosides. Mostly crystalline, less often amorphous substances, highly soluble in water and alcohol. carbohydrate aglycone carbohydrates amorphous substances in alcohol Glycosides are a large group of organic substances found in the plant (less often in the animal) world and/or obtained synthetically. During acid, alkaline, enzymatic hydrolysis, they are split into two or more components - an aglycone and a carbohydrate (or several carbohydrates). Many of the glycosides are toxic or have a strong physiological effect, for example, glycosides of digitalis, strophanthus and others. Most often, glycosides are found in the leaves and flowers of plants, less often in other organs. The composition of glycosides includes carbon, hydrogen, oxygen, less often nitrogen (amygdalin) and only some contain sulfur (sinalbin, myrosin) hydrolysis carbon hydrogen oxygen nitrogen nitrogen (amygdalin) serus albine myrosine

CHEMICAL AND PHYSICAL PROPERTIES OF GLYCOSIDES From the chemical side, glycosides are sugar esters that do not give carbonyl reactions, which means that the carbonyl group of sugars is associated with an aglycone, similar to alkyl glycosides of synthetic glycosides. In glycoside molecules, sugar residues are connected to the aglycone, which is the pharmacologically active part of the glycoside, through the O, N or S atom. Aglycones include for the most part hydroxyl derivatives of the aliphatic or aromatic series. The structure of many natural glycosides is not well understood. By reacting sugars with alcohols, mercaptans, phenols and other substances in the presence of hydrochloric acid, synthetic glycosides are obtained. Compounds of this kind are especially easily formed by the interaction of hydroxyl or other derivatives with acetochloro- or acetobromoglucose. Alcohol mercaptanamiphenols. In the case when glucose is formed during the hydrolysis of glycosides, such compounds are usually called glucosides; when other sugars are formed, glycosides. glucose Glycosides are solid, non-volatile , mostly well-crystallized, less often amorphous substances, easily soluble in water and alcohol. Aqueous solutions of glycosides have a neutral reaction. Although their breakdown into sugars and aglycones occurs very easily, there are also known glycosides (saponins) that are not decomposed even by dilute acids (H 2 SO 4) upon prolonged heating. When glycosides are broken down by enzymes, a certain selectivity is observed; only a certain enzyme is capable of degrading a particular glycoside. Less often, one enzyme breaks down several glycosides; for example, emulsin breaks down not only amygdalin, but also salicin, esculin, coniferin and some other glycosides, but does not break down sinigrin. The yeast enzyme breaks down amygdalin into prunosine; on the contrary, emulsin decomposes it into benzaldehyde cyanohydrin. saponins salicine esculin coniferin sinigrine prunosine

The hydrolyzing effect of enzymes is closely related to the structure of the glycoside molecule and the asymmetry of the carbon atoms of sugars. For example, dextrorotatory α-methyl glucoside is cleaved by invertin, while its levorotatory isomer does not change; on the contrary, β-methyl glucoside is cleaved by emulsin without affecting the α-isomer. Natural glycosides, broken down by emulsin, have left rotation. Enzymes. Partial breakdown of glycosides occurs partly in the plant itself, since the enzyme located in it (albeit in different cells) sometimes comes into contact with it. The same thing, under certain circumstances, happens when plants are dried or glycosides are isolated from them. Therefore, often the glycosides obtained from dried plants differ sharply from the glycosides found in the fresh plant. In a dried plant, enzymes usually do not exhibit their hydrolytic action, but when moistened with water, especially at °C, an intense hydrolysis reaction occurs. At low temperatures, in the presence of moisture, the action of enzymes slows down, and at 0 °C it is almost undetectable. Above 70 °C, on the contrary, inactivation and destruction of enzymes occurs. hydrolysis enzymes Closely related to glucosides, that is, glucose esters, are pentosides or rhamnosides, which upon hydrolysis, along with aglycones, form rhamnose (for example, frangulin, quercetin), rhamnoglucosides , which upon hydrolysis form rhamnose, glucose and other sugars (for example, rutin, hesperidin). rhamnosefrangulin quercetin rutinghesperidin

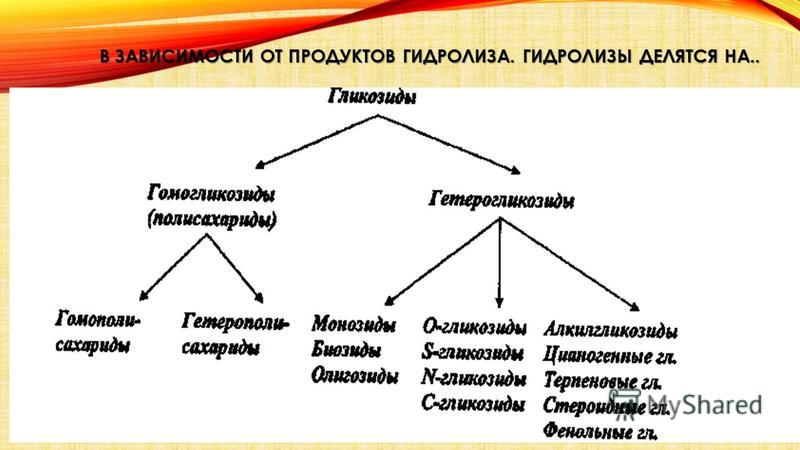
CLASSIFICATION OF GLYCOSIDS The previously very common botanical classification is currently used only for glycosides of unknown structure. Pharmacological classification based on the biological action of glycosides also did not hold up. Most appropriate chemical classification, based on chemical structure aglycones or sugars formed during the hydrolysis of glycosides. In this case, glycosides are called sugars with the addition of the suffix “ide”. Thus, glycosides that cleave off pentose are called pentosides, and glycosides that cleave off hexose are called hexosides. The latter, in turn, are divided into subgroups, for example, those that split off glucose are called glucosides, those that split off fructose or galactose are called fructosides, galactosides, and so on. pentose hexose fructose galactose

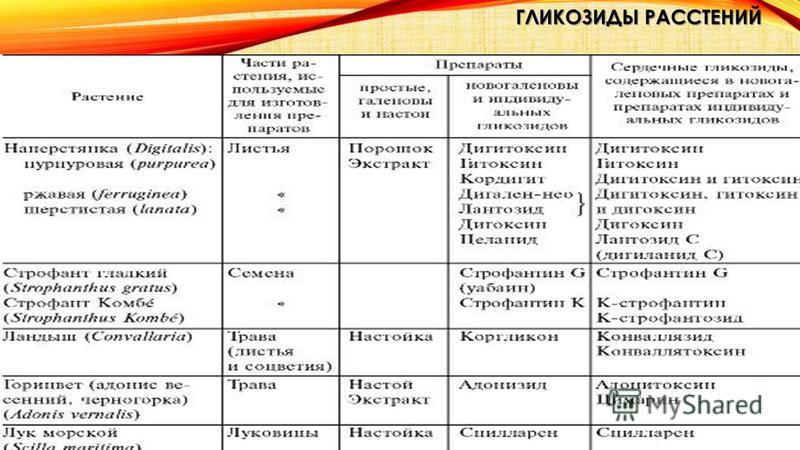
ISOLATION OF GLYCOSIDES FROM PLANTS: Methods for isolating glycosides from plants are very diverse and depend on the nature of the glycosides and their relationship to solvents. Isolation is often very difficult due to their easy decomposition. Usually, when isolating glycosides, the use of acids and alkalis, as well as enzymes that decompose glycosides, is excluded. For this purpose, the plant is treated with alcohol in the presence of alkaline agents (soda, potash, etc.) and then extracted with suitable solvents (water, alcohol, ether, chloroform, dichloroethane, ethyl acetate, etc.) at the appropriate temperature. Sometimes glycosides are converted into insoluble, easily purified compounds and then decomposed in order to isolate them in their pure form. Potassium chloroform and dichloroethane. The crushed plant material is subjected to extraction in diffusers (percolators) and then purification in order to remove tannins, coloring, mucous, protein and other substances, called “ballast”. Due to the usually low content of glycosides in plants, they are often limited to isolating not individual substances, but their mixtures in the form of aqueous solutions, standardized for their biological effect on animals. Such drugs are called neogalenic or novogalenic. Typically, 1 ml of such a solution contains a certain amount of glycosides, expressed in units of action (AU). For example, the activity of cardiac glycosides is expressed in frog (LED) or feline (CED) units, which characterize the smallest amount of a substance that exhibits a biological effect on animals. Naturally, if it is possible to express the activity of glycosides in weight units, the latter are expressed in grams (or milligrams). Particularly great difficulties arise when studying plants in order to search for glycosides. In this case, two main directions are used: the “lead method” or differential sequential extraction. The “lead method” is based on isolating the constituent parts of the plant in the form of lead salts and separating the latter according to their different solubility in certain solvents. With differential extraction, plant material is sequentially extracted with various solvents and chemicals and each extract is studied.

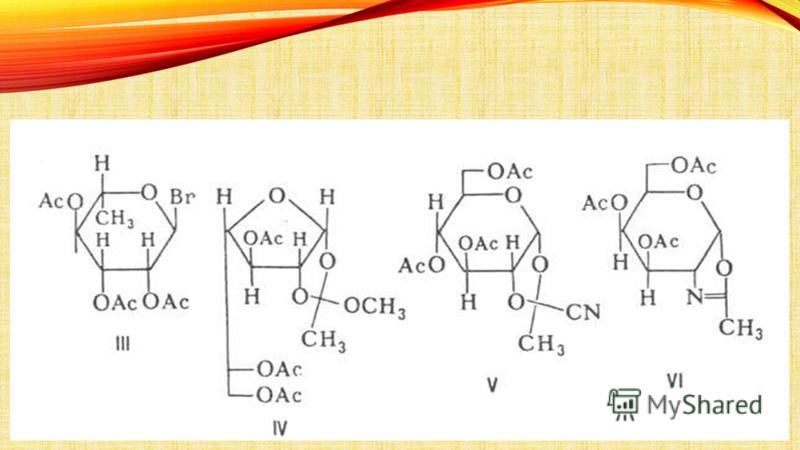
QUALITATIVE REACTIONS OF GLYCOSIDES Glycosides are classified as chemical agents in different ways. Unlike alkaloids, they usually do not give specific reactions; they do not reduce either Fehling's solution or ammonia solution silver oxide. The exception is those glycosides whose aglycones contain reducing groups. After hydrolysis of the glycoside by boiling an aqueous solution with a dilute solution of sulfuric acid, the resulting sugar is detected by its reducing ability with Fehling's solution. Fehling's solution of sulfuric acid with Fehling's solution. More general is enzymatic cleavage, which allows not only to establish the presence of the glycoside, but also to prove its identity by comparison with a known one. This is most often done using the emulsin enzyme. All such glycosides have a left-hand rotation in aqueous solutions, while glucose formed as a result of hydrolysis has a right-hand rotation. Based on these two provisions, each glycoside is characterized by its characteristic enzymolytic recovery index. This index refers to the glucose content, expressed in milligrams in 100 ml of the test solution, resulting from the cleavage of the glycoside in the amount required to change the rotation to the right by 1 ° C in a tube 20 cm long. Color reactions of glycosides are usually suitable only in the absence of free sugars. Thus, many glycosides with purified ox bile and sulfuric acid give a red color, and an alcoholic 20% solution of α-naphthol with concentrated sulfuric acid gives a blue, violet or red color. A similar coloration also occurs when β-naphthol or resorcinol is used. Glycosides containing phenol or compounds with phenolic hydroxyl as an aglycone give a ferric chloride color. With some glycosides, the reaction proceeds more clearly when using alcohol solutions of the reagent. Glycosides whose aglycones contain a carbonyl group are identified as hydrazones, semicarbazones or oximes. When carefully acetylated with acetic anhydride, many glucosides give characteristic acetyl derivatives. The action of an acetylating mixture is sometimes used to discover glucose as a sugar component of a glycoside. Its discovery is based on the conversion of pentaacetylglucose obtained by acetylation into pentaacetylglucosyl-p-toluidide under the action of p-toluidine. This compound is insoluble in alcohol, has a left-hand rotation and has a sharp melting point. hydrazone semi-carbazone oxymovacetic anhydride

SAPONINS Saponins Saponins are complex nitrogen-free organic compounds from glycosides of plant origin with surface active properties. When shaken, solutions of saponins form a thick, persistent foam. The name comes from the Latin sapo (genus saponis) soap. Widely distributed in nature, found in various parts of plants: leaves, stems, roots, flowers, fruits. Contains an aglycone (sapogenin) and a carbohydrate part. surface-active glycosides and soap-aglycone-carbohydrate


ALKALOIDS Alkaloids Alkaloids (from Latin alkali alkali and other Greek ε δος appearance, appearance) are a group of nitrogen-containing organic compounds of natural origin (most often of plant origin), predominantly heterocyclic, most of which have the properties of a weak base; They also include some neutral and even weakly acidic compounds that are biogenetically related to the main alkaloids. Amino acids, nucleotides, amino sugars and their polymers are not alkaloids. Sometimes synthetic compounds of a similar structure are also called alkaloids. Latin alkali Ancient Greek nitrogen-containing organic compounds heterocyclic bases


"Biology. Bacteria, fungi, plants. 6th grade" V.V. Beekeeper
Features of the structure of a plant cell
Question 1. How to prepare a preparation of onion scale skin?
To prepare the onion skin preparation, you need:
1) prepare a glass slide by thoroughly wiping it with gauze;
2) use a pipette to apply 1-2 drops of water onto a glass slide;
3) using a dissecting needle, carefully remove a small piece of transparent skin from the inner surface of the onion scales, put it in a drop of water and straighten it with the tip of the needle;
4) carefully cover the drop of water with the skin with a cover glass.
The drug is ready. It can be viewed through a microscope. You can stain the preparation with iodine solution. For this:
Apply a drop of iodine solution to the slide next to the cover glass so that the iodine solution falls under the cover glass;
on the other side of the cover glass, use filter paper to pull off excess iodine solution
Question 2. What is the structure of a cell?
Each plant cell has a dense transparent shell with pores (Fig. 1). The composition of plant cell walls includes a special substance - cellulose, which gives them strength. Inside the cell there is a colorless viscous substance - cytoplasm. In the cytoplasm there is a small dense nucleus in which the nucleolus can be distinguished. Almost all cells have cavities - vacuoles. They are filled with cell sap. A plant cell is characterized by numerous small bodies - plastids containing pigment. Thus, the green color of leaves is determined by plastids called chloroplasts, which contain the green pigment chlorophyll. The different colors of flower petals are determined by plastids called chromoplasts, which contain red, blue and other colored pigments. For example, the blue color is given by pigment - xanthophyll.
Rice. 1. Structure of a plant cell
Question 3. Where is the cell sap and what does it contain?
Cell sap is found in cell vacuoles. Cell sap contains water with dissolved sugars and other organic and inorganic substances. Cell sap may contain coloring substances - pigments.
Question 4. What color can the dyes found in cell sap and plastids color different parts of plants?
Dyes from cell sap can give blue, purple, and crimson colors to petals and other parts of plants. Plastids can give various plant organs, in particular leaves, green, yellow, red or orange colors. The color of plastids depends on the presence of coloring substances, or pigments. So, the green pigment gives the leaves their green color. chlorophyll.
