Federal agency of Education
State educational institution
Novgorod State University them. Yaroslav the Wise
Faculty natural sciences And natural resources
Department of Chemistry and Ecology
Guidelines
Velikiy Novgorod
Sources of oxygen in water, the effect of oxygen content in water on aquatic organisms, methods for determining oxygen concentration.
The guidelines are intended for students of specialty 020801.65 - “Ecology” and all students studying “General Ecology”.
1 Oxygen in water
Oxygen is one of the most important dissolved gases constantly present in surface waters, the regime of which largely determines the chemical and biological state of water bodies.
1.1 Sources
The main sources of oxygen entering surface waters are the processes of its absorption from the atmosphere and production as a result of the photosynthetic activity of aquatic organisms. Absorption of oxygen from the atmosphere occurs on the surface of the reservoir. The rate of this process increases by lowering the temperature, the degree of saturation of water with oxygen and increasing atmospheric pressure.
The production of oxygen during the process of photosynthesis occurs in the surface layer of the reservoir, the thickness of which depends on the transparency of the water and ranges from several tens of centimeters to several tens of meters.
Oxygen can also enter reservoirs with rain and snow waters, which are usually supersaturated with it.
Aeration - the enrichment of deep layers of water with oxygen - occurs as a result of mixing, including wind, of water masses, vertical temperature circulation, etc.
Oxygen consumption in water is associated with chemical and biochemical processes of oxidation of organic and some Not organic matter(Fe2+, Mn2+, NH+, H2S, CH4, H2, etc.), as well as with the respiration of aquatic organisms. The rate of oxygen consumption increases with increasing temperature, the number of bacteria and other aquatic organisms and substances subject to chemical and biochemical oxidation.
1.2 Forms of migration
Dissolved oxygen in surface waters is in the form of O2 molecules. Its solubility increases with decreasing temperature (Table 1), mineralization (Table 2) and increasing pressure. The solubility of oxygen at normal pressure is called normal concentration. The dependence of normal concentration on temperature is given in table. 1. The saturation of water with oxygen, corresponding to normal concentration, is assumed to be 100%. In the case of high intensity of photosynthesis, water can be significantly oversaturated with oxygen. In this case, part of it can pass into gaseous form.
Table 13 - Dependence of normal oxygen concentration (Co) in water on temperature (Atmospheric pressure 760 mm Hg, partial pressure of oxygen R= 0.209 atm)
Temperature, °C | Dissolved oxygen, mg/l |
|||||||||
Table 14 - Effect of mineralization on the solubility of oxygen in water
In surface waters, dissolved oxygen levels can range from 0 to 14 mg/L and are subject to significant seasonal and diurnal variations. Daily fluctuations mainly depend on the ratio of the intensity of the processes of its production and consumption and can reach 2.5 mg/l of dissolved oxygen. In river waters, the highest concentrations are usually observed in autumn, the lowest in winter, when, as a result of the formation of ice cover, the supply of oxygen from the atmosphere stops. Oxygen deficiency is more often observed in eutrophicated water bodies, as well as in water bodies containing a large number of pollutants and humic substances.
The vertical distribution of oxygen in the absence of intensive mixing of water masses can be quite uneven. Oxygen stratification, like temperature stratification, is most significant in the summer and winter periods. During periods of spring and autumn homothermy, the distribution of oxygen in depth is more uniform.
1.4 Properties, purposes of observation
The oxygen concentration determines the magnitude of the redox potential and, to a large extent, the direction and speed of the processes of chemical and biochemical oxidation of organic and inorganic compounds.
The oxygen regime has a profound impact on the life of a reservoir. The minimum content of dissolved oxygen that ensures normal development of fish is about 5 mg O2/l. Reducing it to 2 mg/l causes mass death of fish. Oversaturation of water with oxygen also has an adverse effect on their condition. The maximum permissible concentration of dissolved oxygen for fishery water bodies is 4 mg/dm3 in winter, and 6 mg/dm3 in summer.
The determination of oxygen in surface waters is included in observation programs in order to assess the living conditions of aquatic organisms, including fish, indirect characteristics of water quality, the intensity of the processes of production and destruction of organic substances, self-purification of water bodies, etc.
Oxygen concentration is expressed either in milligrams per liter or in percent saturation, oxygen is calculated using the formula
DIV_ADBLOCK42">
The above interferences in the analysis of surface waters are usually small and additional operations are required in relatively rare cases.
IN last years Various electrochemical methods for the determination of oxygen have rapidly developed. The main advantages of these methods are their simplicity, low sensitivity to the presence of foreign substances, the possibility of automation and determination of dissolved oxygen in situ. Among the many electrochemical methods, the most widely used are amperometric and polarographic methods using semi-permeable membranes that separate electrodes in an electrolyte solution from the water under study.
Polymer polyethylene and fluoroplastic films, which have satisfactory mechanical properties and high chemical and thermal resistance, are usually used as materials for semi-permeable membranes. In the Soviet Union and abroad, many instruments are currently proposed for electrochemical measurement of dissolved oxygen concentration, which differ in electrode systems, type and design of sensors, membrane materials and the composition of the electrolytes used. Depending on this, the range of the minimum detectable concentration is quite wide (from 0.001 to 1 mg/l).
To monitor the oxygen content in surface waters, iodometric (Winkler) and electrochemical methods are recommended.
2 Safety requirements
The experiments are carried out strictly in accordance with the methodological guidelines. When performing work, you should do general rules safety precautions for chemical laboratories. If reagents come into contact with skin or clothing, the affected area should be quickly rinsed with plenty of water.
3 Experimental part
Goal of the work:
1. Master the method of determining the oxygen content in water.
IODOMETRIC DETERMINATION
Oxygen is an unstable component, the determination of which, due to the dependence of its content on water temperature, must be carried out at the sampling site. The method is intended for the analysis of uncolored or lightly colored waters with an oxygen content above 0.05 mg O2/l.
Principle of the method. The method is based on the interaction of manganese hydroxide with oxygen dissolved in water in an alkaline environment. Manganese hydroxide, quantitatively binding oxygen dissolved in water, turns into insoluble brown tetravalent manganese compounds. When the solution is acidified in the presence of excess potassium iodide, iodine is formed, the amount of which is equivalent to the content of dissolved oxygen and is taken into account by titrating the thiosulfate solution:
Mn2+ + 2OH - → Mn(OH)2 (white);
2Mn(OH)2 + O2 → 2MnO(OH)2 (brown);
MnO(OH)2 + 4H+ + 3I - → Mn2+ + I3- + 3H2O;
I3- + 2S2O32- → 3I - + S4O62-.
Progress of determination. A water sample to determine dissolved oxygen is taken with a bathometer, to the tap of which a rubber tube 20-25 cm long is attached. Oxygen is fixed immediately after sampling. To do this, the oxygen flask is rinsed 2-3 times and then filled with the water to be tested. The rubber tube should touch the bottom of the bottle. After filling the bottle to the neck, continue filling it until approximately 100 ml of water pours out, i.e., until the water in contact with the air in the bottle is displaced. The tube is removed without stopping the flow of water from the bathometer. The bottle should be filled to the brim with sample and not have any air bubbles inside on the walls.
Then, 1 ml of a solution of manganese chloride and 1 ml of an alkaline solution of potassium iodide are introduced into a bottle with a water sample. In this case, it is necessary to use separate pipettes. The pipette is immersed to half the bottle each time and raised upward as the solution is poured out. Then quickly close the bottle with a glass stopper so that there are no air bubbles left in it, and the contents of the bottle are thoroughly mixed.
The resulting precipitate of manganese hydroxide is allowed to settle for at least 10 minutes and no more than a day. Then add 5 ml of HC1 solution. The pipette is immersed to the sediment and slowly raised up. Displacement of part of the clear liquid from the flask with a solution of hydrochloric acid does not matter for analysis.
The bottle is capped and the contents are thoroughly mixed. Take 50 ml of solution with a pipette (the pipette must first be rinsed with this solution) and transfer it to a 250 ml conical flask. The solution is titrated with 0.02 N. thiosulfate solution until it turns light yellow. Then add 1 ml of freshly prepared starch solution and continue titration until the blue color disappears.
Calculation. The mass concentration of oxygen dissolved in water is found using the formula
http://pandia.ru/text/80/154/images/image003_31.gif" width="105" height="47 src=">,
Where CX - found average oxygen concentration, mg/dm3; Sn- normal oxygen concentration taking into account the actual pressure and mineralization of the sample, mg/dm3.
To calculate the normal oxygen concentration in accordance with real atmospheric pressure and salinity, using Table 1, find the equilibrium concentration of dissolved oxygen at the water temperature measured at the time of sampling. From the found equilibrium concentration value, subtract for every 1000 mg/dm3 of salts 0.0840 mg/dm3 at a temperature of 0 °C, 0.0622 mg/dm3 at 10 °C, 0.0478 mg/dm3 at 20 °C and 0. 0408 mg/dm3 at 30 °C. The correction for intermediate values of temperature and salinity is found by interpolation.
Calculation of equilibrium concentration Sn at real pressure is carried out according to the formula
631 " style="width:473.25pt;border-collapse:collapse;border:none">
Measuring range of mass concentration of dissolved oxygen Cx, mg/dm3
Repeatability index σr, mg/dm3
Reproducibility index σR, mg/dm3
Accuracy indicator ±Δ, mg/dm3
From 1.0 to 3.0 incl.
St. 3.0 to 15.0 incl.
The work report must be prepared correctly, accurately, and on time. The report must indicate the sample number and its description (location of the reservoir). The measurement results for each water sample can be presented in the form of a table:
Cx, mg/dm3 | ||
After the table, it is necessary to draw a conclusion about the quality of the studied reservoirs in terms of oxygen content.
5 TEST QUESTIONS
1. On what indicators does the solubility of oxygen in water depend?
2. What are the 2 main methods for determining oxygen concentration?
3. What oxygen concentrations are observed in natural bodies of water?
4. How does a change in oxygen content affect aquatic organisms?
5. What oxygen concentrations cause the death of aquatic organisms?
1 Oxygen in water. 3
1.1 Sources. 3
1.2 Forms of migration. 3
1.4 Properties, purposes of observation. 5
1.5 Determination methods. 6
2 Safety requirements. 7
3 Experimental part. 7
5 Control questions.. 10
Oxygen saturation degree -
physical and chemical indicator of water
Natural water contains dissolved oxygen in the form of O 2 molecules. The processes occurring in water affect the oxygen content in two opposite directions: some contribute to an increase in oxygen concentration, others, on the contrary, lead to its decrease.
The processes of the first group include the following: absorption of oxygen from the atmosphere, photosynthesis, which leads to the release of oxygen by aquatic vegetation, replenishment of reservoirs with rain and snow water, which, as a rule, contains abundant oxygen.
All these processes are not typical for artesian waters, as a result of which there is no oxygen in them. As for surface waters, the oxygen content in them is less than it could theoretically be. This is probably due to processes leading to a decrease in oxygen concentration. Such processes include, first of all, the oxidation reaction and oxygen consumption by various organisms.
 The degree of oxygen saturation is the relative content of oxygen in water, expressed in percentage with its normal content. Factors influencing the degree of oxygen saturation are, first of all, water temperature, salinity level and atmospheric pressure. This parameter is usually calculated using the following formula: M = (ax01308x100)/NxP. Explanation of the formula: M - degree of oxygen saturation (%), a - oxygen concentration (mg/dm 3), N - normal oxygen concentration (at a total pressure of 0.101308 MPa and a given temperature), P - atmospheric pressure. The table below shows normal oxygen concentration in relation to temperature:
The degree of oxygen saturation is the relative content of oxygen in water, expressed in percentage with its normal content. Factors influencing the degree of oxygen saturation are, first of all, water temperature, salinity level and atmospheric pressure. This parameter is usually calculated using the following formula: M = (ax01308x100)/NxP. Explanation of the formula: M - degree of oxygen saturation (%), a - oxygen concentration (mg/dm 3), N - normal oxygen concentration (at a total pressure of 0.101308 MPa and a given temperature), P - atmospheric pressure. The table below shows normal oxygen concentration in relation to temperature:

Parameters such as the value of the redox potential, the direction and speed of the processes of chemical and biochemical oxidation of organic and inorganic compounds. Determining the degree of oxygen saturation of surface waters allows for an additional assessment of water quality. Below is a table illustrating the classification of reservoirs according to this indicator:

The World Health Organization does not propose any standards regarding the oxygen content of water based on its effect on human health. However, a sharp decrease in oxygen indicates chemical or biological contamination. Depletion of dissolved oxygen in water supplies can cause the microbiological reduction of nitrate to nitrite and sulfate to sulfide, which in turn leads to odor. Another consequence of low oxygen content in water is an increase in the concentration of ferrous iron in the solution and, as a result, difficulties in removing it.
Under certain conditions, dissolved oxygen can give water corrosive properties relative to metal and concrete.
The normal degree of oxygen saturation for surface waters is reduced to at least 75%.
Ministry of Education and Science of the Russian Federation
State educational institution of higher professional education
"Ufa State Petroleum Technical University"
Department of “Applied Ecology” determination of dissolved oxygen and biochemical oxygen consumption in water
EDUCATIONAL MANUAL
to perform laboratory work
Laboratory work is intended primarily for students of the specialty “Environmental Protection and Rational Use of Natural Resources” when studying the disciplines “Environmental Monitoring” and “Industrial Ecology”. Can be used for all specialties in the disciplines “Ecology” and “Industrial Ecology”. When using the “RK-BPK” test kit, intended for express analysis in samples of surface water, land, standard-treated waste and drinking water, students can determine these water quality indicators while undergoing educational practice at the educational, research and production site of USPTU .
Compiled by: F.A. Shakhova, Candidate of Chemical Sciences, Associate Professor of the Department. PE
I.F. Fakhretdinova, student of gr. OS-05-01
A.I. Mukhamadeeva, student of gr. OS-05-01
Dissolved oxygen (dc)
Oxygen is constantly present in dissolved form in surface waters. The content of dissolved oxygen (DO) in water characterizes the oxygen regime of a reservoir and is of utmost importance for assessing its ecological and sanitary condition. Oxygen must be contained in water in sufficient quantity, providing conditions for the breathing of aquatic organisms. It is also necessary for self-purification of reservoirs, because. participates in the processes of oxidation of organic and other impurities, decomposition of dead organisms. A decrease in DO concentration indicates a change in biological processes in the reservoir, and pollution of the reservoir with biochemically intensely oxidizing substances (primarily organic). Oxygen consumption is also due to chemical processes of oxidation of impurities contained in water, as well as the respiration of aquatic organisms.
Oxygen enters a reservoir by dissolving it upon contact with air (absorption), as well as as a result of photosynthesis by aquatic plants, i.e. as a result of physicochemical and biochemical processes. Oxygen also enters water bodies with rain and snow water. Therefore, there are many reasons that cause an increase or decrease in the concentration of dissolved oxygen in water.
Oxygen dissolved in water is in the form of hydrated molecules ABOUT 2 . The DO content depends on temperature, atmospheric pressure, degree of water turbulization, amount of precipitation, water salinity, etc. At each temperature value, there is an equilibrium oxygen concentration, which can be determined using special reference tables compiled for normal atmospheric pressure. The degree of saturation of water with oxygen, corresponding to the equilibrium concentration, is taken equal to 100%. Oxygen solubility increases with decreasing temperature and mineralization and with increasing atmospheric pressure.
In surface waters, dissolved oxygen levels can range from 0 to 14 mg/L and are subject to significant seasonal and diurnal variations. In water bodies that are eutrophicated and heavily polluted with organic compounds, there may be a significant oxygen deficiency. A decrease in DO concentration to 2 mg/l causes mass death of fish and other aquatic organisms.
In the water of reservoirs at any time of the year until 12 noon, the DO concentration must be at least 4 mg/l. The maximum permissible concentration of oxygen dissolved in water for fishery reservoirs is set at 6 mg/l (for valuable fish species) or 4 mg/l (for other species).
Dissolved oxygen is a highly unstable component chemical composition water When determining it, sampling should be carried out especially carefully: it is necessary to avoid contact of water with air until oxygen is fixed (binding it into an insoluble compound).
Control of oxygen content in water is an extremely important problem, the solution of which is of interest to almost all sectors of the national economy, including ferrous and non-ferrous metallurgy, the chemical industry, agriculture, medicine, biology, the fishing and food industries, and environmental protection services. The DO content is determined both in unpolluted natural waters and in wastewater after treatment. Wastewater treatment processes are always accompanied by oxygen content control. The determination of DO is part of the analysis when determining another important indicator of water quality - biochemical oxygen demand (BOD).
The concentration of dissolved oxygen in water is a consequence of two opposite and simultaneously occurring processes:
oxygen consumption by organic substances (natural and coming from wastewater);
atmospheric reaeration as the most significant source of oxygen supply to the reservoir.
(The amount of oxygen produced by plants depends on many factors that are difficult to take into account and becomes negligible during periods of weak vegetation development.)
The interaction between these two processes is illustrated by the figure (see below).
We examined the features of the process of oxygen consumption in the previous section.
Let's move on to reaeration.
Water is considered saturated with oxygen if it contains it within the limits of its solubility. The difference between the amount of oxygen at complete and actual saturation is called oxygen deficiency D (in mg/l or %).
Reaeration of water bodies obeys the following law: the rate of oxygen dissolution is directly proportional to the degree of undersaturation of water, i.e. oxygen deficiency.
Oxygen deficiency after a certain period of time D t will be equal to:
where D o is the initial oxygen deficiency, k 2 is the reaeration constant.
The rate of saturation of water with oxygen depends on:
Oxygen deficiency in the surface layer of water;
The size of the surface in contact with the atmosphere in relation to the volume of water in the reservoir;
Mixing intensity.
Therefore, k 2 is different for reservoirs with different hydrological regimes. There are no laboratory methods for determining k2. It can be established as a result of observations in reservoirs and solving the equation of the oxygen deflection curve (Fig.) relative to k 2. therefore, the approximate values given in the table are usually used. Moreover, they try not to use higher limits of reaeration constants for greater reliability of calculations (calculation is carried out for the worst conditions).
Equations (69) and (80) separately characterize the processes of oxygen consumption and reaeration. The oxygen regime of a reservoir is a consequence of the combined influence of these processes. It became possible to determine this influence after deriving the Phelps-Streeter equations (81-82), which takes into account both factors:
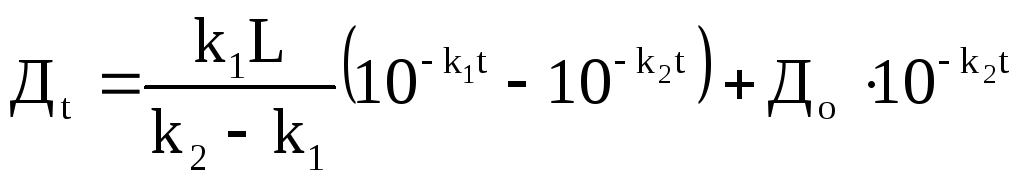 (81)
(81)
where L is the average calculated BOD value of the mixture of wastewater and reservoir water, calculated only taking into account the dilution process (the self-purification factor is taken into account in the Phelps-Streeter equation) according to the formula:
Equation (81) describes the oxygen deflection curve. By calculating D t at any point below the wastewater discharge and knowing the value of complete oxygen saturation (O us), you can calculate the content of oxygen dissolved in water (O fact) using the equation:
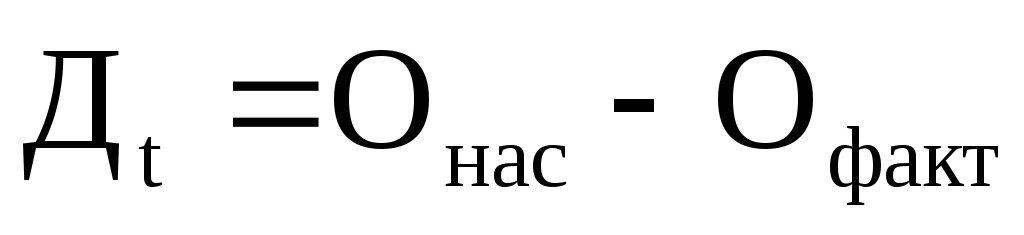 (84)
(84)
The calculation is based on the following premises:

The calculation algorithm is constructed as follows:
First, tcr is calculated,
Then Dcr is determined, for time t = tcr,
Determine the estimated oxygen content in the water body O fact according to the equation:

 (86)
(86)
If O fact ≥ 4, as required by sanitary standards, then the calculation made using BOD also ensures the required oxygen regime of the reservoir.
A simpler approach is based on the idea of wastewater absorbing oxygen from river water and only to a certain extent takes into account the possibility of reaeration. The calculation is based on the assumption that the maximum oxygen deficiency (D cr) is usually observed during the first two days:
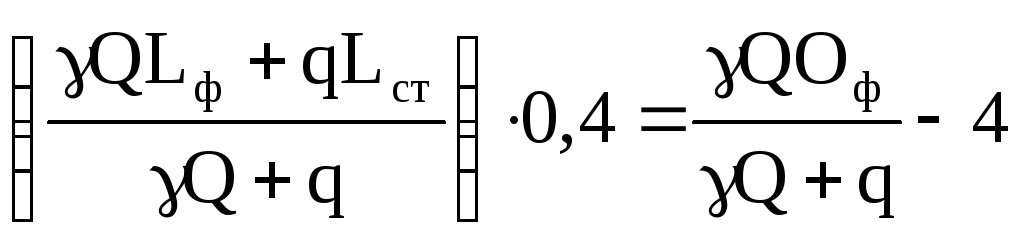 (87)
(87)
where Of is the background oxygen content in the water body; 0.4 – conversion factor of BOD total to BOD 2 (since the maximum oxygen deficiency is observed in the first two days);
4 – 4 mg/l of oxygen should be contained in the water of a water body according to sanitary requirements, i.e. This is the oxygen standard.
The right side of equation (78) is the maximum permissible oxygen consumption, which will not threaten the oxygen regime of the reservoir. On the left side of equation (87) in parentheses is a balance formula that allows you to calculate the total BOD in a reservoir, which will be created as a result of wastewater discharge, given using a coefficient of 0.4 to BOD 2. Thus, the biochemical oxygen consumption for 2 days (the left side of equation 87) should not exceed the permissible oxygen consumption (the right side of equation 87).
Let's solve equation (87) for L st, which will now make sense  , i.e. maximum permissible BOD of wastewater, calculated taking into account regulatory requirements for dissolved oxygen content:
, i.e. maximum permissible BOD of wastewater, calculated taking into account regulatory requirements for dissolved oxygen content:
 (88)
(88)
If the calculated BOD concentration () turns out to be less than the actual BOD value in the wastewater, then the wastewater must be further purified. The cleaning efficiency is determined by formula (79).
The need to calculate the possibility of discharging wastewater both according to BOD and dissolved oxygen is due to the fact that local conditions that allow the possibility of discharging wastewater according to the dissolved oxygen rate may turn out to be completely unsuitable for discharging wastewater with given BOD values. This is especially true when the water temperature is low and reaeration occurs.
Therefore, you first need to calculate by BOD, and only then by dissolved oxygen.
When wastewater enters containing individual substances limited by the general sanitary water limit (for example, substances that have a bactericidal effect and thereby inhibit biochemical processes: Cd, Cu, Zn, Ni, etc.), the calculation of the possibility of draining wastewater is carried out in the same way as for individual toxic substances.
Dissolved oxygen is in natural water in the form of O2 molecules. Its content in water is affected by two groups of oppositely directed processes: some increase the oxygen concentration, others reduce it. The first group of processes that enrich water with oxygen includes: the process of absorption of oxygen from the atmosphere; release of oxygen by aquatic vegetation during photosynthesis; entry into reservoirs with rain and snow waters, which are usually oversaturated with oxygen. Absorption of oxygen from the atmosphere occurs at the surface water body. The rate of this process increases with decreasing temperature, increasing pressure and decreasing mineralization. Aeration - enrichment of deep layers of water with oxygen - occurs as a result of mixing of water masses, including circulation, etc. Photosynthetic release of oxygen occurs when carbon dioxide is assimilated by aquatic vegetation (plants and phytoplankton). The rate of oxygen consumption increases with increasing temperature, the number of bacteria and other aquatic organisms and substances subject to chemical and biochemical oxidation. The oxygen concentration determines the magnitude of the redox potential and, to a large extent, the direction and speed of the processes of chemical and biochemical oxidation of organic and inorganic compounds. The oxygen regime has a profound impact on the life of the aquarium. Active aeration of the water in the aquarium through continuously operating air sprays or filter ejectors should ensure maximum saturation of the water with oxygen.
Water is a good solvent. Generally speaking, most of free oxygen enters water from the atmosphere, although in the daytime some of it can be provided by plants. Water can absorb oxygen from the air only where these two elements border, namely on the surface of the water. Similarly, carbon dioxide is released into the atmosphere only on the surface of the water. The larger the water surface area, the more oxygen it can absorb and the more carbon dioxide highlight. This fact is very important for keeping fish, since the amount of oxygen determines the number of fish that a given aquarium can support, as well as for choosing the optimal shape of the aquarium. In an aquarium, the enrichment of water with gases occurs through the surface as a result of the activity of hydrobionts and with the help of special technical devices (aerators, filters). The transition of gases through the surface occurs due to molecular diffusion; When air bubbles pass through the filter and aerator spray, the same molecular diffusion operates. Roughness on the surface of the water increases its effective surface area. A surface covered with ripples has a larger area than a flat surface, thereby increasing its ability to exchange gases. Water circulation is also very useful, since it brings water rich in carbon dioxide to the surface, and carries water that has just been saturated with oxygen to the bottom layer. Aquarists call the combined process of surface movement and water circulation aeration.
The top layer of water in the aquarium is mostly saturated with this gas. Therefore, in order to distribute oxygen evenly, it is necessary to maintain constant vertical rotation of the water using an aerator or filter. Species-specific oxygen requirements generally depend on the oxygen content of their natural habitat. For example, fish from biotopes with a high oxygen content - such as fast-moving rivers or large, windy lakes - require more oxygen than those living in slow-moving water. Individual fish typically have an increased oxygen requirement when they feel unwell, are stressed, are more active than usual (for example, during spawning or when they are being chased), or if they are kept at a higher temperature than nature intended. Likewise, fish require less oxygen when they are inactive (for example, diurnal fish at night) or if the water temperature is lower than necessary. The hydrobionts inhabiting the aquarium have different attitudes towards the saturation of water with oxygen. Based on their oxygen requirements, fish are usually divided into four groups:
1. Fish of cold and fast rivers, the so-called rheophilic: sturgeon, some species of catfish, goby, found in aquariums.
2. Fish that live in rivers and creeks, lakes, and slow-flowing waters - the majority of aquarium fish.
3. Fish of stagnant waters - from goldfish and its varieties to the Amur eleotris or rotan, which is extremely undemanding in terms of oxygen content.
4. Fish that have additional respiratory organs that allow them to capture atmospheric air.
For the correct maintenance of most fish, it is necessary to follow a regime that satisfies the fish of the second group. There are no fundamental differences in the transportation of gases by blood in fish. As in pulmonary animals, in fish the transport functions of the blood are realized due to the high affinity of hemoglobin for oxygen, the relatively high solubility of gases in the blood plasma, and the chemical transformation of carbon dioxide into carbonates and bicarbonates. The main transporter of oxygen in the blood of fish is hemoglobin. The diffusion of oxygen from water into the blood occurs along a concentration gradient. The gradient is maintained when oxygen dissolved in plasma is bound by hemoglobin, i.e. diffusion of oxygen from water occurs until hemoglobin is completely saturated with oxygen. In the vast majority of fish, gas exchange without hemoglobin is practically impossible. In fish that live in an environment rich in oxygen, and in our case we will talk about them, hemoglobin can bind oxygen only when there is a significant amount of it. Studies carried out when growing carp showed that the increased oxygen content in the water significantly improved their general condition, increased appetite and immunity to diseases, improved growth and weight especially in young fish, enhanced the sexual function of adult fish, improved the digestibility of feed and metabolism, and had a positive effect on blood composition. At the same time, the aquariums must have clean, turbid-free water, a sufficient number of well-lit aquatic plants, constant mechanical mixing of the water with an aerator and filtration. The amount of oxygen consumed by fish is not stable. A decrease in oxygen concentration affects the development of fish; Their appetite usually does not decrease, but the biological direction of the digested food changes, less nutrients are absorbed, and as a result, growth slows down. Taking this into account, when juveniles are densely planted in nursery aquariums, it is necessary to ensure constant water exchange and aeration.
Fish, like people, breathe oxygen, which they extract from water, and exhale carbon dioxide. Most aquarium fish and aquatic plants breathe oxygen dissolved in water. Only a small part of fish are able to partially use atmospheric oxygen for breathing. In nature, they usually live in bodies of water where there is a seasonal or constant lack of oxygen. These are labyrinth fish (gourami, lalius, etc.) that swallow atmospheric air into a special organ - a labyrinth, where gas exchange occurs. The most interesting way breathing is described in some armored catfish (for example, catfish - cockroaches). It turns out that they swallow a bubble of atmospheric air, pass it into the intestines, and there gas exchange occurs between the body and the external environment. Aquatic plants (including algae) absorb carbon dioxide during the daytime or when the lights are on in the aquarium. They use the carbon it contains to produce nutrients and release free oxygen into the water. However, at night they absorb oxygen and release carbon dioxide. The oxygen consumed by fish and plants is necessary to oxidize organic compounds in cells and provide them with energy. If the process of gas exchange between the body and the external environment is disrupted, then the animal dies quite quickly. Thus, providing the fish in the aquarium with the oxygen necessary for breathing is a prerequisite.
Lake cichlids, especially the inhabitants of the lake. Tanganyika has fairly stringent requirements for the composition and purity of water. Excessive content of decaying organic matter leads to the formation of toxic compounds that cause disease and death of fish. Optimal conditions: hardness 10-20°, pH 8-9, temperature 24-27°C. Higher temperatures are unacceptable (especially long-term). Continuous filtration and aeration of water are required. The inhabitants of lakes Malawi and Victoria are not so demanding on the composition of the water; they have a higher adaptability to unfavorable conditions. I think there is no point in talking about the need for a thermostat or thermometer in an aquarium with Tanganyika cichlids. But aeration using a compressor still requires two words. Large fish release quite a lot of organic matter and nitrogen, and consume large amounts of oxygen. Therefore, filtration and aeration must be powerful and effective. Even if your filter successfully aerates the water, a good additional blowing point through the porous stone will not hurt, but will enhance the filter aeration system.
The most important thing for fish is oxygen dissolved in water. The concentration of oxygen dissolved in water is directly dependent on the population of the aquarium, its depth, water surface area, lighting conditions, water temperature and some other factors. Aquatic plants play a huge role in maintaining normal oxygen conditions in the aquarium. Its content varies depending on temperature (as it decreases, the solubility of oxygen increases, and vice versa), atmospheric pressure (the higher the pressure, the greater the solubility of oxygen), the intensity of water mixing, the presence of phytoplankton and higher aquatic plants.
Gas exchange is a very important process for the fish body. The oxygen content in water is 20 times less than in air. There are 2 ways to saturate water with oxygen: mechanical using a compressor and biological - the release of oxygen by aquatic plants. Aquarium plants must be illuminated for photosynthesis to occur and oxygen to be released. The lower permissible limit of oxygen content is 3-5 mg/l, the upper limit is 15 mg/l, and fish living in an aquarium with a very fast flow consume more oxygen. The aquarium should contain oxygen between 5 mg/l in the morning and 10 mg/l in the evening. In an aquarium, a value of 5 mg/l of oxygen is taken as a minimum, that is, this value should be understood as the limiting value necessary for breathing. If it is lower, many fish may suffocate. This limit value is not sufficient for the entire biological system of the aquarium to function correctly. Often fish suffer from a lack of oxygen, which can be caused by overcrowding of the aquarium or its overload with oxidizing organic substances. Too many plants can also lead to a lack of oxygen. Fish that are not getting enough oxygen tend to move their gills too often, open their mouths, and concentrate near the surface of the water, where the oxygen content is higher.
In an aquarium with a small number of inhabitants and if there are plants in it, as a rule, there is no need to saturate the water with oxygen, since it is formed in sufficient quantities as a result of photosynthesis. In an aquarium with a lot of living plants, aeration is mandatory. However, in practice there is usually not enough oxygen. Therefore, the water needs to be aerated by blowing air through it from sprayers, pumped using a compressor. If the water in the aquarium is artificially heated, then aeration is also necessary, even if there are few fish in a large vessel, not so much to saturate the water with oxygen, but to mix it with air bubbles in order to reduce sudden changes in water temperature, both horizontally and vertically. We should not forget that the higher the temperature, the less oxygen dissolves in it. The use of air bubbles generated by a spray from a compressor can also serve additional element decorating the aquarium. Vertically rising bubbles decorate the aquarium, creating a certain movement of the glass reservoir. With bubbles, the aquarium looks more lively and attractive. Many aquarium fish love to frolic in bubbles. In many of the proposed decorations - grottoes with sandy waterfalls, in opening chests, in everything spinning or tilting, an air lift is used (creating a flow of water using air bubbles).
The lack of oxygen dissolved in water causes mass death of fish - so-called death. In addition, unfavorable zoohygienic conditions are created in the reservoir: organic substances accumulate and saprophytic microflora multiply, which can have a negative effect on fish. Oxygen starvation leads to illness and death of fish. Fish that spend a long time in water with insufficient oxygen levels experience decreased activity, become lethargic, consume little food, become exhausted, and their overall resistance to adverse environmental factors and pathogens of infectious diseases is significantly reduced.
What factors influence the oxygen saturation of water?
The oxygen content in water is affected by the temperature of the water: the warmer the water, the less oxygen it contains, and vice versa. In addition, increased temperature accelerates metabolic processes in fish, as a result of which their need for oxygen increases precisely at a time when its content in the water decreases. Of course, the water temperature, the higher it is, the worse the oxygen supply. A factor that influences the physical, chemical, biochemical and biological processes occurring in the aquarium, on which the oxygen regime and the intensity of self-purification processes largely depend. Temperature values are used to calculate the degree of oxygen saturation of water, various forms alkalinity, the state of the calcium carbonate system, in many hydrochemical and hydrobiological studies. Temperature is measured using thermometers, and thermostats set the required mode.
As temperature increases, the solubility of oxygen in water decreases. IN fresh water oxygen concentration at +15°C = 9.9 mg/l, at +20°C = 8.9 mg/l, at +25°C = 8.1 mg/l, at +30°C = 7, 4 mg/l. The oxygen regime of a reservoir also depends on the content of organic substances in the water. The more of them there are in water, the more oxygen is consumed for their oxidation during the decomposition process, because When rotting occurs, oxygen is used, therefore, the less oxygen remains in the water, which is necessary for fish to breathe. This problem can be overcome by more intensive aeration.
However, let us recall here that strong saturation of water with oxygen leads to an increase in pH, which is undesirable both from the point of view of the requirement for consistency in the parameters of aquarium and replacement water, and from an increase in the percentage of non-ionized ammonia in water with a high pH. The oxygen entering the volume during aeration serves not only for the respiration of fish and microorganisms, but is the most important factor in reducing ballast and some harmful substances in the aquarium. In order for the supplied oxygen to be evenly distributed throughout the volume of the aquarium, a naturally intensive water circulation is necessary.
Plants are often valued for their ability to produce oxygen. However, it should be remembered that at night they themselves consume oxygen and produce carbon dioxide. Thus, although plants can indeed help meet the oxygen needs of fish during the day, at night all living things in the aquarium compete for oxygen, the content of which decreases at this time of day. Therefore, in aquariums that are densely planted, there may be a lack of oxygen at night. In recent years, some aquarists have been using carbon dioxide to speed up plant growth. At the same time, it is introduced into the aquarium from special cylinders. However, the introduction of CO2 should be done with great caution, and perhaps should not be done at all. Don't forget that high level CO2 content can lead to a decrease in the amount of oxygen absorbed by the water, and then there is a risk of hypoxia in fish - especially if the aquarium is densely populated or there are fish among those that have a high need for oxygen. Some aquarists try to get around this problem by using additional aeration. However, although this measure will certainly increase the oxygen content and benefit the fish, it will also promote the removal of carbon dioxide, and this will make the addition of CO2 completely pointless. The CO2 generator must be turned off at night when plants do not need this gas.
A large snail population can have a significant impact on the oxygen content of an aquarium. Bacteria can do the same. Oxygen consumption by aerobic bacteria involved in the nitrogen cycle is acceptable because they provide significant benefits in return. However, if there is excess organic waste in the aquarium (for example, due to regularly overfeeding the fish), the bacterial population will grow and absorb more oxygen than if the fish are fed rationally. Snails, of course, also increase organic waste.
The concentration of oxygen in aquarium water depends on how balanced the aquarium is. It is necessary that the density of the fish population in the aquarium matches its volume, and that there is also a good filtration system. In this case, there is no need to install a compressor to blow air into the aquarium, since the filtration unit will ensure complete mixing of the aquarium water, and oxygen will be supplied in the required amount from the surrounding air by diffusion. Insufficient water aeration, excess nitrogen compounds and free organic matter in the water in the form of food residues and fish secretions (if you have insufficient filtration and irregular water changes, as well as frequent overfeeding of fish), abuse of feeding aquatic plants with carbon dioxide, etc.
If there is not enough oxygen in the water, then carbon dioxide, released by fish during respiration, quickly accumulates in the environment, and they die from suffocation - fish death. The prerequisites for death are too high density fish, water blooms, increased water temperature in the aquarium (in summer on hot days or if the aquarium has a poor-quality thermostat), as well as the use of certain medications. The aquarist's reaction in these cases should be very quick and correct. The first thing to do is quickly install additional aeration. If there is a power outage in your home for quite a long period of time, you need to purchase a battery-powered compressor. If you have too many fish in your aquarium (for example: at first you planted fry, but then they grew and the biomass became too large, or some fish multiplied directly in the aquarium), then you need to remove some of the fish to another aquarium. Excessive gases in the water can also lead to the death of fish due to gas embolism. This disease occurs when water is oversaturated with gases, resulting in the formation of bubbles directly in the water. The formation of gas bubbles on the surface and inside the body of fish is very dangerous, and gas bubbles in the blood lead to blockage of blood vessels, i.e. to embolism. The fish die a painful death. Most often, this disease is observed when unsettled tap water is added to the aquarium. An excess amount of gases is dissolved in it, which immediately begins to form bubbles on glass, plants, and fish. There are known cases of this disease manifesting itself during water blooms, as well as during increased aeration of aquariums with a large number of plants during a period of high photosynthesis intensity. However, it is quite easy to protect yourself from gas embolism: you need to add only well-settled water to the aquarium and do not over-aerate the water.
Aquariums in which the height significantly exceeds the width, the so-called screens, look beautiful, especially when they contain tall plants and tall fish (for example, angelfish). The depth of the water to the ground should not exceed 50 cm. The height of the aquarium reduces the intensity of light required to illuminate the entire aquarium, and, accordingly, problems with algae fouling of glass, plants and decorations. In tall aquariums, the lower layers of water are poorly saturated with oxygen, which leads to mandatory aeration of the water with a compressor. The higher the aquarium, the more powerful the compressor is required to create more pressure. For aquariums larger than 60 cm, it is difficult, and for aquariums larger than 70 cm, it is impossible to reach objects with your hand from the bottom of an aquarium filled with water. This leads to great difficulties in caring for it.
The more blue-green algae there are, the more alkaline the environment. Soft water with little bicarbonate content has an unstable pH value. Moreover, in real practice, alkalization of water is observed extremely rarely, but a decrease in pH below the permissible level is very easy to achieve. To reduce sharp fluctuations, it is recommended that the water be aerated at night with air, which will carry carbon dioxide with it.
To create normal gas exchange between water and air, the surface of the water must be in constant movement. Aquariums in which a film of bacteria and dust forms on the surface cannot be sufficiently supplied with oxygen.
Flow and aeration of aquarium water.
If we want to recreate in an aquarium the natural conditions for the biotopes of our ornamental fish, then we must proceed from the fact that most fish live in flowing - fast or moderate - water. Even if the main part of the flow is characterized by high speed, fish still live in areas where resistance to fast water does not require much effort from them. Aeration should be carried out using a filtration system designed in such a way as to ensure maximum water circulation and, if possible, disturbance on the surface of the water. Aeration can also be done using an air pump (microcompressor and sprayer). This creates a flow of air bubbles, which also promotes the circulation of water and the formation of ripples on its surface. Contrary to popular belief, air bubbles themselves add relatively little oxygen to the water. It is their effect on water circulation and surface gas exchange that brings benefits. In most of our aquariums, the flow created by the filter provides the necessary water movement. Whether the electric pump takes over the transportation of water or the airlift does it is a secondary matter. In practice, there are two methods of directing filter water into the aquarium: it is sprayed above the water surface over as wide a surface area as possible (and the water can absorb a fairly large amount of oxygen) or an electric pump forces the water coming from the filter inside the tank itself, below the surface. In the second case, the water cannot receive air from the surface, and then this the most important process- oxygen access must be achieved in a different way. A diffuser is a small device, most often made of organic glass or other plastic, which is usually installed at the filter drain. A nipple connection on its outside allows you to connect an air hose to it. Due to the flow of filtration water under pressure, outside air enters through the air hose and, together with the filtration water, is forced into the aquarium water. The diffuser creates turbulence and the air bubbles gently burst. They are very small and light, and therefore do not rise to the surface very quickly. In addition, using a clamp that regulates the pressure, you can reduce or increase, and, if necessary, dose the air supply.
Aeration of water in an aquarium is one of the vital conditions for the normal existence of living organisms in an aquarium, especially when there are a significant number of them. Aeration promotes a favorable gas exchange regime in the aquarium, saturating the water with the necessary amount of oxygen. During aeration, vortex currents are formed on the surface of the water, which facilitate the absorption of oxygen and the removal of carbon dioxide. Fish breathe oxygen and release carbon dioxide, which is consumed by plants during photosynthesis, which in turn release oxygen. If the ratio of the number of fish and plants in the aquarium is correctly chosen, then these gases are enough for them, and they grow and develop well. The smaller the bubbles, the greater the total surface area and the better the enrichment of water with oxygen. Gas exchange, that is, the enrichment of water with oxygen and the removal of carbon dioxide, occurs mainly at its surface, where air bubbles carry along the lower, oxygen-poor layers of water. It also occurs through the walls of the bubbles themselves, for which they strive to make them as small as possible: with increasing common surface gas exchange conditions improve. It is especially important to carry out aeration at night, since the process of photosynthesis occurs only in light during the day, and at night there may come a time when there is an excess of carbon dioxide and a lack of oxygen in the water. Periodic, short-term, so-called pumping of air into the aquarium does little good - it can even harm fish and plants, causing sudden changes in the amount of oxygen in the aquarium and disrupting their vital functions.
But if in densely populated aquariums the organic content is very high, then in decorative aquariums this factor, within reasonable limits, can be neglected. However, in such aquariums, significant deposition of organic matter also occurs, both in the soil and in the prefilters (mechanical cleaning compartments). Deposits are especially visible on the sponges of internal filters and other similar systems. Most aquarists use so-called external filters. But basically they can be considered as purely mechanical filters. The reason is that they cannot actively supply themselves with oxygen. Biological filtration in an aerobic environment is associated specifically with oxygen, which enters closed filters only with aquarium water. In aquariums that use such filters, intensive water aeration is important. Organic matter formed inside the filter cavities requires significant oxygen consumption, therefore, enriching water with oxygen is necessary not only to supply animals with it, but also to replenish the oxygen spent on the oxidation of organic matter. Closed-type filters have a drawback: if there is not enough oxygen for nitrification, and the content of organic substances exceeds permissible standards, then the process proceeds unsatisfactorily and the filter can “topple” into an anaerobic environment.
You can notice signs of excess ammonia and its compounds, overload with proteins and their breakdown products, by the formation of persistent foam from the operation of the air spray. The air bubbles that rise to the surface should burst within 1-3 seconds. If this does not happen, then there is an excess of them in the aquarium. Often the main source of ammonia is a filter that has not been cleaned for a long time. You need to know that you can bring the concentration of nitrogen-containing toxins to a critical point very quickly in 1-2 days. To reduce the toxicity of ammonia, four rules should be followed: constant aeration, cleanliness in the aquarium; regular water changes, moderate population of plants and animals. To limit the content of nitrates, regular water changes and planting are required, and excess plants must be removed.
Feeding units on weekends and holidays should be located in the area of water flow from the filter water pump or near the compressor nozzle. Then the separated particles will be spread throughout the aquarium, otherwise they will only be consumed by bottom-dwelling fish. You can place them at a distance of 5-7 cm from the surface of the water (preferably in a net for catching fish), this will provide food for fish of all layers, and will also get rid of such a scourge as shellfish, because especially melania, stick around the block so tightly that the fish simply have nowhere to stick. White blocks are gypsum. These blocks increase the pH as the gypsum dissolves and are not suitable for fish that require acidic water.
