Born in 1844 in Vienna. Boltzmann is a pioneer and pioneer in science. His works and studies were often incomprehensible and rejected by society. However, with the further development of physics, his works were recognized and subsequently published.
The scientific interests of the scientist covered such fundamental areas as physics and mathematics. Since 1867, he worked as a teacher in a number of higher educational institutions. In his studies, he found that it is due to the random impact of molecules on the walls of the vessel in which they are located, while the temperature directly depends on the speed of movement of the particles (molecules), in other words, on them. Therefore, the more these particles move, the higher the temperature. Boltzmann's constant is named after the famous Austrian scientist. It was he who made an invaluable contribution to the development of static physics.
The physical meaning of a given constant
The Boltzmann constant determines the relationship between such as temperature and energy. In static mechanics, it plays a major key role. The Boltzmann constant is equal to k \u003d 1,3806505 (24) * 10 -23 J / K. The numbers in parentheses indicate the permissible error of the value of the quantity relative to the last digits. It is worth noting that the Boltzmann constant can also be obtained from other physical constants. However, these calculations are quite complicated and difficult to perform. They require deep knowledge not only in the field of physics, but also
−1
Boltzmann's constant ( k (\\ displaystyle k) or k B (\\ displaystyle k _ (\\ rm (B)))) - a physical constant that defines the relationship between temperature and energy. Named after the Austrian physicist Ludwig Boltzmann, who made a great contribution to statistical physics, in which this constant plays a key role. Its experimental value in the International System of Units (SI) is:
k \u003d 1,380 648 52 (79) × 10 - 23 (\\ displaystyle k \u003d 1 (,) 380 \\, 648 \\, 52 (79) \\ times 10 ^ (- 23)) J /.The numbers in parentheses indicate the standard error in the last digits of the value.
The relationship between temperature and energy[ | ]
In a homogeneous ideal gas at absolute temperature T (\\ displaystyle T), the energy attributable to each translational degree of freedom is equal, as follows from the Maxwell distribution, k T / 2 (\\ displaystyle kT / 2). At room temperature (300), this energy is 2, 07 × 10 - 21 (\\ displaystyle 2 (,) 07 \\ times 10 ^ (- 21)) J, or 0.013 eV. In a monatomic ideal gas, each atom has three degrees of freedom corresponding to three spatial axes, which means that each atom has energy in 3 2 k T (\\ displaystyle (\\ frac (3) (2)) kT).
Knowing the thermal energy, we can calculate the root-mean-square velocity of atoms, which is inversely proportional to the square root of the atomic mass. The rms velocity at room temperature varies from 1370 m / s for helium to 240 m / s for xenon. In the case of a molecular gas, the situation becomes more complicated, for example, a diatomic gas has five degrees of freedom (at low temperatures, when atomic vibrations in the molecule are not excited).
Entropy Definition[ | ]
The entropy of a thermodynamic system is defined as the natural logarithm of the number of different microstates Z (\\ displaystyle Z)corresponding to a given macroscopic state (for example, a state with a given total energy).
S \u003d k ln \u2061 Z. (\\ displaystyle S \u003d k \\ ln Z.)Proportionality coefficient k (\\ displaystyle k) and there is a Boltzmann constant. This expression defines the relationship between microscopic ( Z (\\ displaystyle Z)) and macroscopic states ( S (\\ displaystyle S)), expresses the central idea of \u200b\u200bstatistical mechanics.
The basic equation of molecular kinetic theory ideal gas carries a deeper meaning than the usual formula for determining the ideal gas pressure. To find out, we write this equation p \u003d (1/3). n m 0v̅ 2 in a slightly different form:
p \u003d (2/3). n. m 0v̅ 2/2 \u003d (2/3). nE̅.
A-priorym 0v̅ 2/2is the average kinetic energy of the translational motion of the molecule.
The gas pressure is proportional to the average kinetic energy of the translational motion of the molecules.
Whereas n \u003d N /V \u003dN A /V m, the equation p \u003d (2/3). nE̅ will look like:
pV M \u003d (2/3). N A E̅.
On the other hand, from the equation of state of an ideal gas
pV M \u003d RT.
Comparing both of these equations, we get:
(2 / 3) . N A E̅ \u003dRT.
E̅ \u003d (3/2). (R /N A) . T.
An important conclusion follows from this relation:
the average kinetic energy of gas molecules is directly proportional to its absolute temperature.
Universal gas constant ratio R to constant Avogadro N a there is also a constant, which is called boltzmann constant k.
R /N A \u003dk - boltz mana constant.
Physical meaning boltzmann constant consists in the fact that it establishes the ratio of temperature, expressed in energy (J) and thermodynamic (K) units.
Boltzmann constant is a fundamental constant, the value of which is determined quite accurately:
k \u003d 1.38. 10 -23 J/ K.
E̅ \u003d (3/2). kT.
If this expression is substituted in the formula p \u003d (2/3). nE̅, we obtain the dependence of the ideal gas pressure on temperature and the concentration of its molecules:
p \u003dnkT.
This relationship is confirmed experimentally charles law, according to which the pressure of a given mass of gas is directly proportional to the absolute temperature: p ~T. It also follows from it that
at the same pressure and temperature, the concentration of molecules in all gases is the same.
Thus, temperature as a macro-parameter of a system characterizes the state of its thermodynamic equilibrium. Material from the site
The approach of the body temperature to absolute zero leads to a decrease in the average kinetic energy of the molecules. At absolute zero, their translational motion ceases. Modern science denies the possibility of achieving absolute zero temperatures.
Depending on the chosen scale, the temperature is measured in degrees Celsius or Fahrenheit or in kelvins. As a micro-parameter of a system, temperature determines the average kinetic energy of a significant number of molecules; as her measure she is measured in joules. The coupling coefficient between these definitions is boltzmann constant.
Despite the fact that the conclusion that the temperature is related to the average kinetic energy of the molecules is established for gases, it is also valid for liquids and solids.
On this page, material on the topics:
Molecular-kinetic interpretation of the phenomenon of osmosis
Constants in molecular kinetic physics
Molecular kinetic interpretation of the temperature of the formula
-
Boltzmann Ludwig (1844-1906)- The great Austrian physicist, one of the founders of molecular-kinetic theory. In the works of Boltzmann, molecular-kinetic theory first appeared as a logically coherent, consistent physical theory. Boltzmann gave a statistical interpretation of the second law of thermodynamics. He did a lot to develop and popularize the theory of electromagnetic field Maxwell. A wrestler by nature, Boltzmann passionately defended the need for a molecular interpretation of thermal phenomena and took on the brunt of the struggle with scientists who denied the existence of molecules.
Equation (4.5.3) includes the ratio of the universal gas constant R to constant Avogadro N A . This ratio is the same for all substances. It is called the Boltzmann constant, in honor of L. Boltzmann, one of the founders of the molecular-kinetic theory.
Boltzmann constant is equal to:
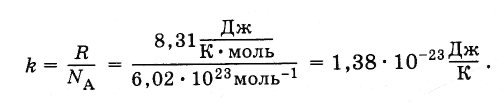 (4.5.4)
(4.5.4)Equation (4.5.3) taking into account the Boltzmann constant is written as follows:
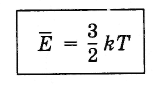 (4.5.5)
(4.5.5)The physical meaning of the Boltzmann constant
Historically, temperature was first introduced as a thermodynamic quantity, and a unit of measure was set for it - degree (see § 3.2). After establishing the relationship of temperature with the average kinetic energy of the molecules, it became apparent that the temperature can be defined as the average kinetic energy of the molecules and expressed in joules or ergs, i.e. instead of Tenter value T *so that
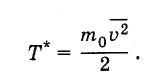
The temperature thus determined is related to the temperature, expressed in degrees, as follows:
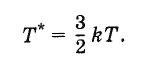
Therefore, the Boltzmann constant can be considered as a value connecting the temperature, expressed in energy units, with the temperature, expressed in degrees.
Dependence of gas pressure on the concentration of its molecules and temperature
Expressing Efrom relation (4.5.5) and substituting into formula (4.4.10), we obtain an expression showing the dependence of gas pressure on the concentration of molecules and temperature:
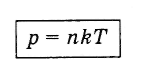 (4.5.6)
(4.5.6)It follows from formula (4.5.6) that, at the same pressures and temperatures, the concentration of molecules for all gases is the same.
From here follows the Avogadro law: equal volumes of gases at the same temperatures and pressures contain the same number of molecules.
The average kinetic energy of the translational motion of molecules is directly proportional to absolute temperature. Proportionality coefficient- boltzmann constantk \u003d 10 -23 J / K - need to remember.
§ 4.6. Maxwell distribution
In a large number of cases, knowledge of the average values \u200b\u200bof physical quantities alone is not enough. For example, knowledge of the average height of people does not allow planning production of clothes of various sizes. You need to know the approximate number of people whose growth lies in a certain interval. Likewise, it is important to know the numbers of molecules having velocities other than the mean. Maxwell was the first to find how these numbers can be determined.
Probability of a random event
In §4.1, we already mentioned that to describe the behavior of a large set of molecules J. Maxwell introduced the concept of probability.
As was repeatedly emphasized, it is in principle impossible to trace the change in the speed (or momentum) of one molecule over a long time interval. It is also impossible to accurately determine the velocities of all gas molecules at a given time. From the macroscopic conditions in which the gas is located (a certain volume and temperature), certain values \u200b\u200bof the molecular velocities do not follow with necessity. The speed of a molecule can be considered as a random variable, which under various macroscopic conditions can take different values, just as when throwing a dice any number of points from 1 to 6 can drop out (the number of bone faces is six). It is impossible to predict how many points will fall during a given dice. But the likelihood that, say, five points will be dropped, can be determined.
What is the probability of a random event? Let a very large number be produced Ntest (N is the number of bone rolls). Moreover, in N" in cases there was a favorable outcome of the tests (i.e., loss of the five). Then the probability of this event is equal to the ratio of the number of cases with a favorable outcome to the total number of trials, provided that this number is arbitrarily large:
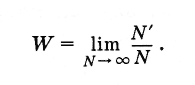 (4.6.1)
(4.6.1)For a symmetrical bone, the probability of any selected number of points from 1 to 6 is equal.
We see that against the background of many random events a certain quantitative regularity is revealed, a number appears. This number - probability - allows you to calculate averages. So, if you make 300 bone rolls, then the average number of five drops, as follows from formula (4.6.1), will be: 300 · \u003d 50, and it makes no difference to throw the same bone 300 times or 300 identical bones at the same time .
There is no doubt that the behavior of gas molecules in a vessel is much more complicated than the movement of an abandoned dice. But here one can also hope to find certain quantitative regularities that make it possible to calculate statistical averages, if only the task is set in the same way as in game theory, and not as in classical mechanics. It is necessary to abandon the insoluble problem of determining the exact value of the molecule’s speed at a given moment and try to find the probability that the speed has a certain value.
